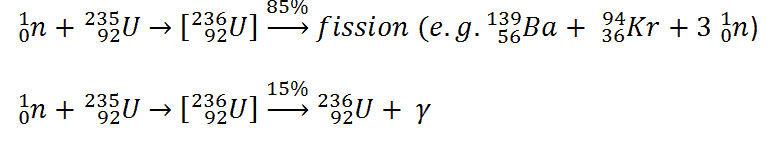In physics, there are two very important principles concerning the electric charge.
First is the law of conservation of electric charge. This law states that:
The algebraic sum of all the electric charges in any closed system is constant.
The only way to change the net charge of a system is to bring in charge from elsewhere or remove a charge from the system.
The charge can be created and destroyed, but only in positive-negative pairs.
Conservation of charge is thought to be a universal conservation law. No experimental evidence for any violation of this principle has ever been observed. In particle physics, charge conservation means that an elementary particle reactions that create charged particles, equal numbers of positive and negative particles are always created, keeping the net amount of charge unchanged. Even in high-energy interactions in which particles are created and destroyed, such as the creation of positron-electron pairs, the total charge of any closed system is exactly constant.
The second important principle is:
The magnitude of the charge of the electron or proton is a natural unit of charge.
We say that charge is quantized. That is, every observable amount of electric charge is always an integer multiple of this basic unit. This unit is called the elementary charge, e, approximately equal to 1.602×10−19 coulombs (except for particles called quarks, which have charges that are integer multiples of 1⁄3e).
Conservation of Charge in Nuclear Reactions
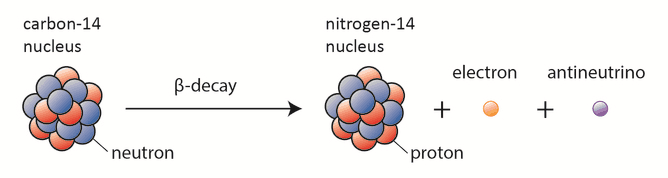
Important examples of the law of conservation of electric charge occur in nuclear reactions such as the radioactive decay of nuclei and nuclear fission. In these reactions, a nucleus transforms into a different type of nucleus or nuclei.
Consider a typical fission reaction such as the one listed below.
Typically, when uranium 235 nucleus undergoes fission, the nucleus splits into two smaller nuclei (triple fission can also rarely occur), along with a few neutrons (the average is 2.43 neutrons per fission by thermal neutron) and release of energy in the form of heat and gamma rays. From these reactions, we find that in the fission, the parent nucleus:
- 235U contains 92 protons (a charge of +92e),
- incident neutron is electrically neutral.
The fission fragments:
- 139Ba contains 56 protons (a charge of +56e),
- 94Ba contains 36 protons (a charge of +36e)
We see that the total charge is 92e before and after the reaction; thus, the charge is conserved. It is noteworthy, the total number of nucleons before and after a reaction are also the same.
Conservation of Charge in Positron-Electron Pair Production
The law of conservation of electric charge can also be demonstrated on positron-electron pair production. Since a gamma-ray is electrically neutral and some of the electric charges of electron and positron are also zero, the electric charge in this reaction is also conserved.
Ɣ → e– + e+
It must be added, for electron-positron pair production to occur, the electromagnetic energy of the photon must be above threshold energy, which is equivalent to the rest mass of two electrons. The threshold energy (the total rest mass of produced particles) for electron-positron pair production equals 1.02MeV (2 x 0.511MeV) because the rest mass of a single electron is equivalent to 0.511MeV of energy. If the original photon’s energy is greater than 1.02MeV, any energy above 1.02MeV is, according to the conservation law, split between the kinetic energy of motion of the two particles. The presence of an electric field of a heavy atom such as lead or uranium is essential to satisfy the conservation of momentum and energy. The atomic nucleus must receive some momentum to satisfy both conservations of momentum and energy. Therefore a photon pair production in free space cannot occur.
Conservation of Electric Charge in Neutrino Discovery
In 1931, Pauli proposed that another particle must be emitted in the beta decay process because the electrons emitted in beta decay have a continuous rather than a discrete spectrum appeared to contradict conservation of energy, under the then-current assumption that beta decay is the simple emission of an electron from a nucleus. This other particle was later named by Fermi the neutrino. It was clear, this particle must be highly penetrating and that the conservation of electric charge requires the neutrino to be electrically neutral. This would explain why it was so hard to detect this particle. The term neutrino comes from Italian, meaning “little neutral one,” and neutrinos are denoted by the Greek letter ν (nu). In the process of beta decay, the neutrino carries the missing energy, and also, in this process, the law of conservation of energy remains valid.