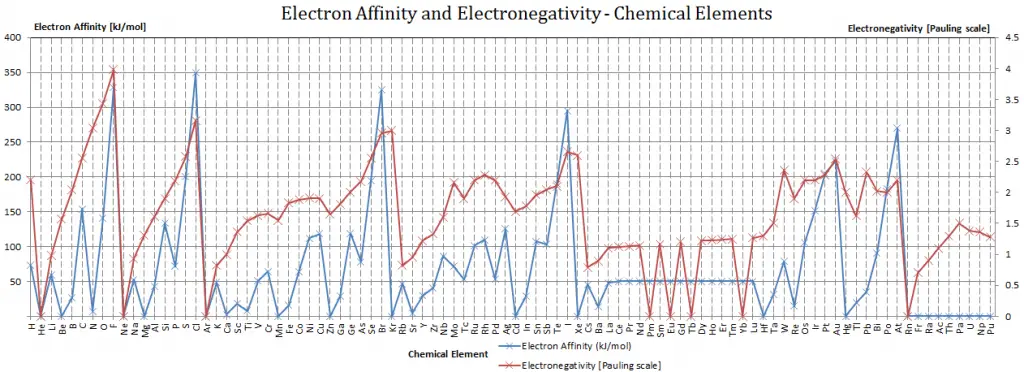
The electronegativity of fluorine is:
χ = 4.0
An atom’s electronegativity is generally affected by its atomic number and the distance its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons toward it.
As it is usually calculated, electronegativity is not a property of an atom alone but rather a property of an atom in a molecule. Even so, the electronegativity of an atom is strongly correlated with the first ionization energy and negatively correlated with the electron affinity. Electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong, attractive force on electrons. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on the negative electrons. Therefore the electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left.
Cesium is the least electronegative element (0.79); fluorine is the most (3.98).
Electronegativity in the periodic table
For full interactivity, please visit material-properties.org.