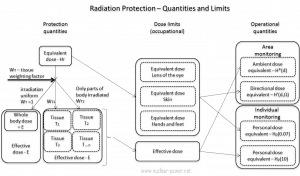
Article Summary & FAQs
What is ionizing radiation?
Ionizing radiation is composed of particles or electromagnetic waves that create the ionizing effect. The kinetic energy of particles (photons, electrons, etc.) of ionizing radiation is sufficient, and the particle can ionize (to form ion by losing electrons) target atoms to form ions.
Key Facts
- Ionizing radiation has different ionization mechanisms and may be grouped as:
- Directly ionizing. Charged particles (atomic nuclei, electrons, positrons, protons, muons, etc.) can ionize atoms directly through fundamental interaction through the Coulomb force if they carry sufficient kinetic energy.
- Alpha radiation. Alpha radiation consists of alpha particles at high energy/speed. The production of alpha particles is termed alpha decay.
- Beta radiation. Beta radiation consists of free electrons or positrons at relativistic speeds. The production of beta particles is termed beta decay.
- Indirectly ionizing. Indirect ionizing radiation is electrically neutral particles and therefore does not interact strongly with matter.
- Photon radiation (Gamma rays or X-rays). Photon radiation consists of high-energy photons. According to the currently valid definition, X-rays are emitted by electrons outside the nucleus, while the nucleus emits gamma rays. The production of gamma rays is termed gamma decay.
- Neutron radiation. Neutron radiation consists of free neutrons at any energies/speeds. This type of radiation can be produced by nuclear reactors or in flight. Neutrons contribute 40 – 80% of the equivalent dose.
- Directly ionizing. Charged particles (atomic nuclei, electrons, positrons, protons, muons, etc.) can ionize atoms directly through fundamental interaction through the Coulomb force if they carry sufficient kinetic energy.
- There are three main types of radiation detectors, which record different types of signals.
- Counter. The activity or intensity of radiation is measured in counts per second (cps).
- Radiation Spectrometer. Spectrometers are devices designed to measure the spectral power distribution of a source.
- Dosimeter. A radiation dosimeter is a device that measures exposure to ionizing radiation.
- In general, there are two broad categories of radiation sources:
- Natural Background Radiation. Natural background radiation includes radiation produced by the Sun, lightning, primordial radioisotopes or supernova explosions, etc.
- Man-Made Sources of Radiation. Man-made sources include medical uses of radiation, residues from nuclear tests, industrial uses of radiation, etc.
What is Radiation
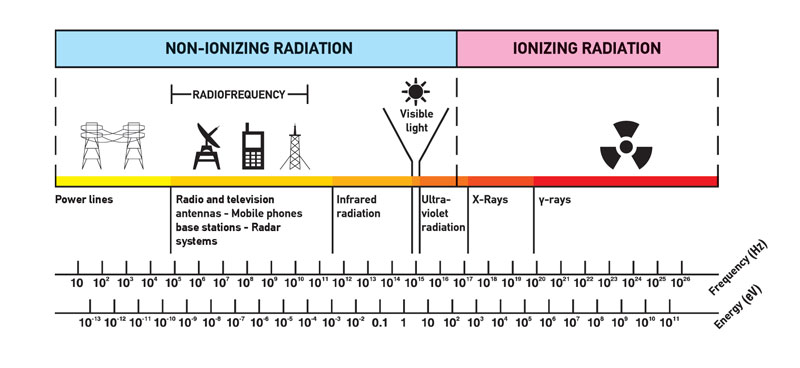
The most general definition is that radiation is energy that comes from a source and travels through some material or through space. Light, heat, and sound are types of radiation. This is a very general definition, the kind of radiation discussed in this article is called ionizing radiation. Most people connect the term radiation only with ionizing radiation, but it is not correct. Radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us. It is a part of our natural world that has been here since the birth of our planet. We should distinguish between:
- Non-ionizing radiation. The kinetic energy of particles (photons, electrons, etc.) of non-ionizing radiation is too small to produce charged ions when passing through matter. The particles (photons) have sufficient energy to change the rotational, vibrational, or electronic valence configurations of target molecules and atoms. Sunlight, radio waves, and cell phone signals are examples of non-ionizing (photon) radiation. However, it can still cause harm, like when you get a sunburn.
- Ionizing radiation. The kinetic energy of particles (photons, electrons, etc.) of ionizing radiation is sufficient, and the particle can ionize (to form ion by losing electrons) target atoms to form ions. Simply ionizing radiation can knock electrons from an atom.
The boundary is not sharply defined since different molecules and atoms ionize at different energies. This is typical for electromagnetic waves. Among electromagnetic waves belong to increasing frequency (energy) and decreasing wavelength: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. Gamma rays, X-rays, and the higher ultraviolet part of the spectrum are ionizing, whereas the lower ultraviolet, visible light (including laser light), infrared, microwaves, and radio waves are considered non-ionizing radiation.
Forms of ionizing radiation
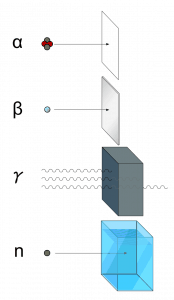
Ionizing radiation is categorized by the nature of the particles or electromagnetic waves that create the ionizing effect. These particles/waves have different ionization mechanisms and may be grouped as:
- Directly ionizing. Charged particles (atomic nuclei, electrons, positrons, protons, muons, etc.) can ionize atoms directly through fundamental interaction through the Coulomb force if they carry sufficient kinetic energy. These particles must be moving at relativistic speeds to reach the required kinetic energy. Even photons (gamma rays and X-rays) can ionize atoms directly (despite they are electrically neutral) through the Photoelectric effect and the Compton effect, but secondary (indirect) ionization is much more significant.
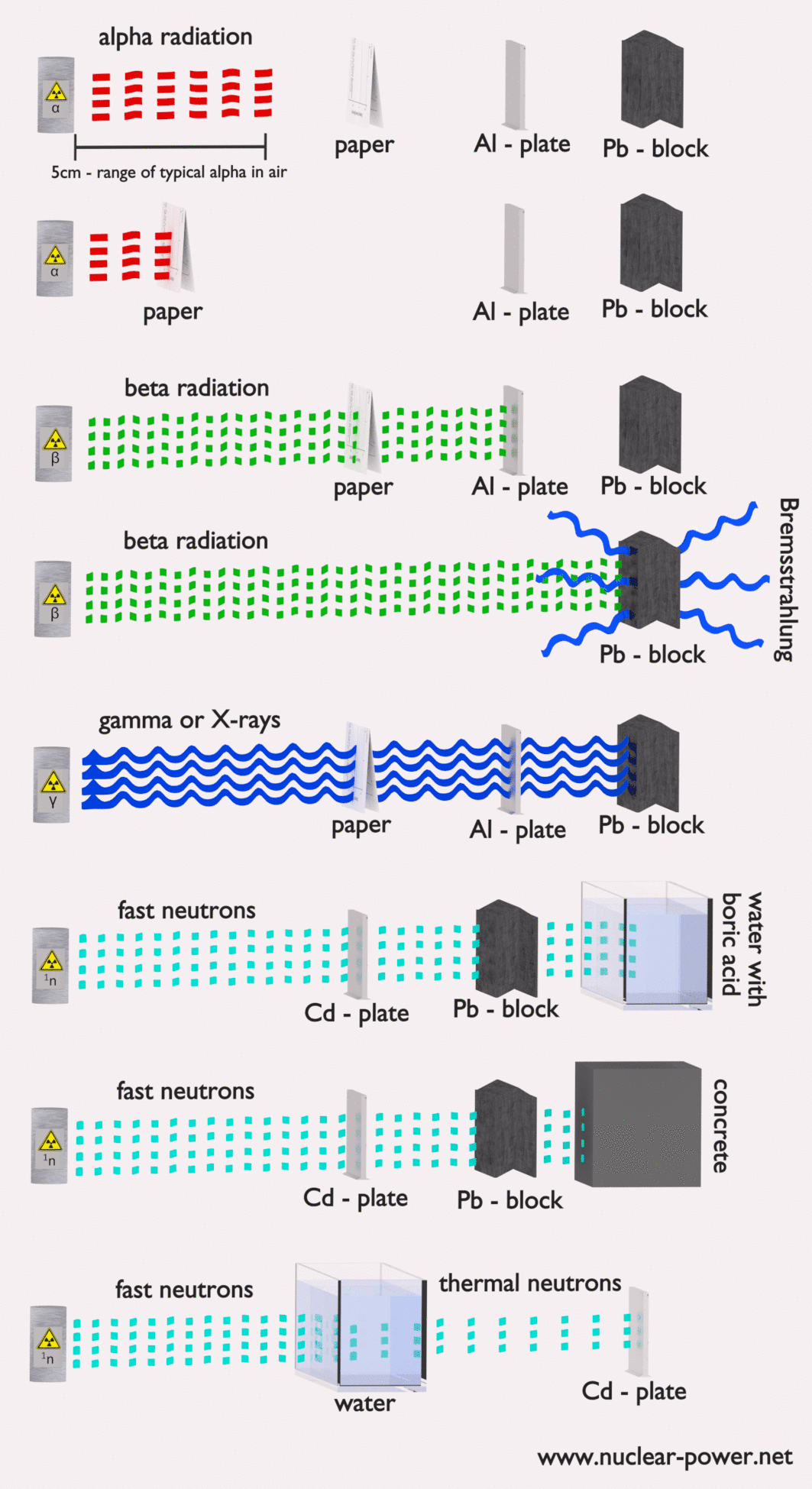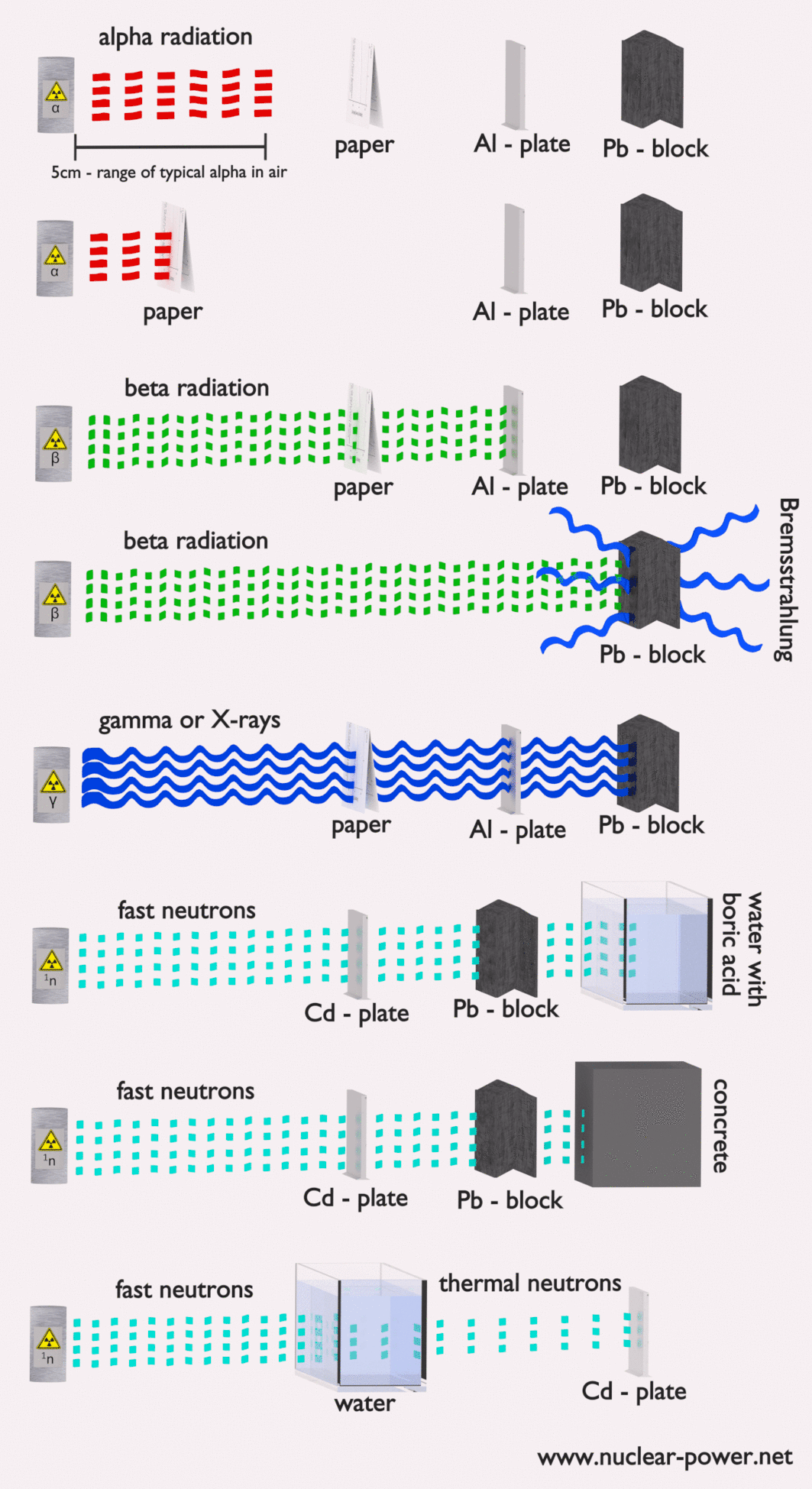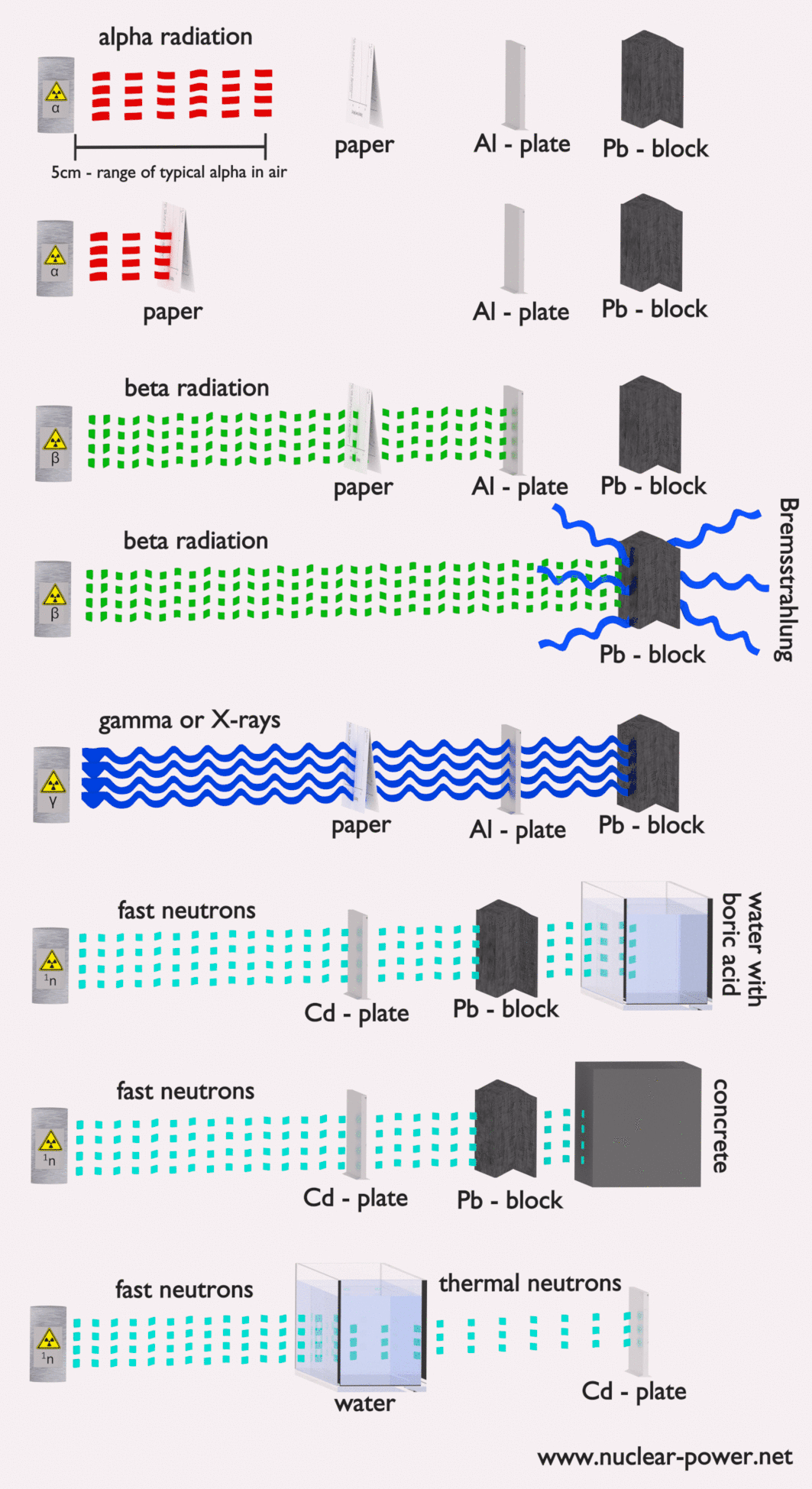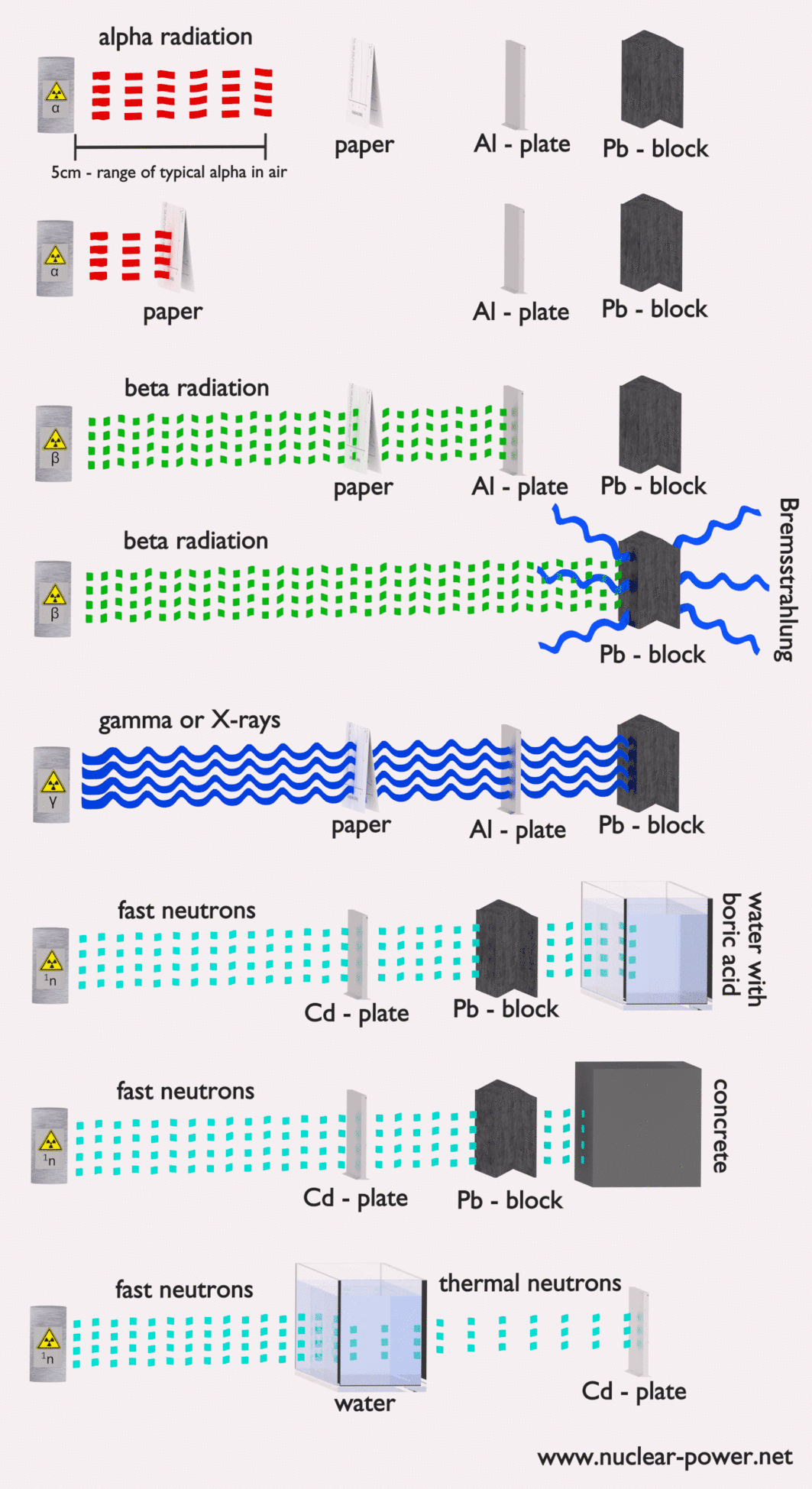
- Alpha radiation. Alpha radiation consists of alpha particles at high energy/speed. The production of alpha particles is termed alpha decay. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Alpha particles are relatively large and carry a double positive charge. They are not very penetrating, and a piece of paper can stop them. They travel only a few centimeters but deposit all their energies along their short paths.
- Beta radiation. Beta radiation consists of free electrons or positrons at relativistic speeds. Beta particles (electrons) are much smaller than alpha particles. They carry a single negative charge. They are more penetrating than alpha particles, but thin aluminum metal can stop them. They can travel several meters but deposit less energy at any point along their paths than alpha particles.
- Indirectly ionizing. Indirect ionizing radiation is electrically neutral particles and therefore does not interact strongly with matter. The bulk of the ionization effects are due to secondary ionizations.
- Photon radiation (Gamma rays or X-rays). Photon radiation consists of high-energy photons. These photons are particles/waves (Wave-Particle Duality) without rest mass or electrical charge. They can travel 10 meters or more in the air. This is long-distance compared to alpha or beta particles. However, gamma rays deposit less energy along their paths. Lead, water, and concrete stop gamma radiation. Photons (gamma rays and X-rays) can ionize atoms directly through the Photoelectric and Compton effect, where the relatively energetic electron is produced. The secondary electron will go on to produce multiple ionization events. Therefore the secondary (indirect) ionization is much more significant.
- Neutron radiation. Neutron radiation consists of free neutrons at any energies/speeds. Neutrons can be emitted by nuclear fission or by the decay of some radioactive atoms. Neutrons have zero electrical charges and cannot directly cause ionization. Neutrons ionize matter only indirectly. For example, when neutrons strike the hydrogen nuclei, proton radiation (fast protons) results. Neutrons can range from high speed, high energy particles to low speed, low energy particles (called thermal neutrons). Neutrons can travel hundreds of feet in the air without any interaction.
Shielding of Ionizing Radiation
Radiation shielding simply means having some material between the source of radiation and you (or some device) that will absorb the radiation. The amount of shielding required, the type of material of shielding strongly depends on several factors. We are not talking about any optimization.
In fact, in some cases, inappropriate shielding may even worsen the radiation situation instead of protecting people from the ionizing radiation. Basic factors, which have to be considered during the proposal of radiation shielding, are:
- Type of the ionizing radiation to be shielded
- The energy spectrum of the ionizing radiation
- Length of exposure
- Distance from the source of the ionizing radiation
- Requirements on the attenuation of the ionizing radiation – ALARA or ALARP principles
- Design degree of freedom
- Other physical requirements (e.g.,, transparence in case of leaded glass screens)
See also: Shielding of Ionizing Radiation
- Shielding of Alpha Radiation
- Shielding of Beta Radiation
- Shielding of Positrons
- Shielding of Gamma Radiation
- Shielding of Neutrons
Shielding in Nuclear Power Plants
Generally, in the nuclear industry, radiation shielding has many purposes. In nuclear power plants, the main purpose is to reduce the radiation exposure to persons and staff in the vicinity of radiation sources. In NPPs, the main source of radiation is conclusively the nuclear reactor and its reactor core. Nuclear reactors are, in general, powerful sources of an entire spectrum of types of ionizing radiation. Shielding used for this purpose is called biological shielding.
But this is not the only purpose of radiation shielding. Shields are also used in some reactors to reduce the intensity of gamma rays or neutrons incident on the reactor vessel. This radiation shielding protects the reactor vessel and its internals (e.g.,, the core support barrel) from excessive heating due to gamma-ray absorption fast neutron moderation. Such shields are usually referred to as thermal shields.
See also: Neutron Reflector.
A little strange radiation shielding is usually used to protect the material of reactor pressure vessel (especially in PWR power plants). Structural materials of pressure vessel and reactor internals are damaged especially by fast neutrons. Fast neutrons create structural defects, which in result lead to embrittlement of material of pressure vessel. To minimize the neutron flux at the vessel wall, the core loading strategy can also be modified. In the “out-in” fuel loading strategy, fresh fuel assemblies are placed at the periphery of the core. This configuration causes high neutron fluence at the vessel wall. Therefore, many nuclear power plants have adopted the “in-out” fuel loading strategy (with low leakage loading patterns – L3P). In contrast to the “out-in” strategy, low leakage cores have fresh fuel assemblies in the second row, not at the periphery of the core. The periphery contains fuel with higher fuel burnup and lowers relative power, and a very sophisticated radiation shield.
In nuclear power plants, the central problem is to shield against gamma rays and neutrons because the ranges of charged particles (such as beta particles and alpha particles) in the matter are very short. On the other hand, we must deal with shielding all types of radiation because each nuclear reactor is a significant source of all types of ionizing radiation.