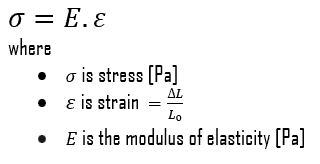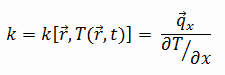High-speed steels, abbreviated as HSS, are a specialized class of tool steels named primarily for their ability to machine and cut materials at high speeds (high hot hardness). It is often used in power-saw blades and drill bits. High-speed steel is superior to the older high-carbon steel tools in that it can withstand higher temperatures without losing its temper (hardness). High-speed steels are complex iron-base alloys of carbon, chromium, vanadium, molybdenum, tungsten, or combinations thereof. An appropriate hardening response must be provided in heat treatment to achieve good cutting performance from HSS.
Central to the performance of high-speed steels is the hardening response achieved during the heat treatment process. Alloying elements are introduced in quantities given by the intended application and their function in the heat treatment process, whether to increase the solidus temperature or inhibit the growth of secondary hardening precipitates, enabling higher operating temperature.
High-speed Steel – AISI M2
For example, molybdenum high-speed steel – AISI M2 is the “standard” and most widely used industrial HSS. According to the AISI classification system, molybdenum high-speed steels are designated as Group M steels. M2 HSS has small and evenly distributed carbides giving high wear resistance, though its decarburization sensitivity is a little bit high. It is usually used to manufacture various tools, such as drill bits, taps, and reamers.
The carbon and alloy contents are balanced at sufficient levels to provide a high attainable hardening response, excellent wear resistance, high resistance to the softening effects of elevated temperature, and good toughness for effective use in industrial cutting applications. Titanium nitride (an extremely hard ceramic material) or titanium carbide coatings can be used in the tools made of this kind of steel through a physical vapor deposition process to improve the performance and life span of the tool. TiN has a Vickers hardness of 1800–2100 and a metallic gold color.
Alloying Agents in High-speed Steels

Pure iron is too soft to be used for the purpose of a structure. Still, adding small quantities of other elements (carbon, manganese, or silicon, for instance) greatly increases its mechanical strength. The synergistic effect of alloying elements and heat treatment produces various microstructures and properties. The four major alloying elements that form carbides in high-speed steels are tungsten, chromium, vanadium, and molybdenum. These alloying elements combine with carbon to form hard and wear-resistant carbide compounds. The microstructure of high-speed steels consists of a martensitic matrix with a dispersion of two sets of carbides. These carbides are usually known as primary and secondary carbides. Primary carbides are those carbides formed during the solidification of the steel, and secondary carbides are those formed during secondary hardening heat-treatment of the steels.
- Tungsten. Produces stable carbides and refines grain size to increase hardness, particularly at high temperatures. Tungsten is used extensively in high-speed tool steels and has been proposed as a substitute for molybdenum in reduced-activation ferritic steels for nuclear applications. Adding about 10% of tungsten and molybdenum efficiently maximizes the hardness and toughness of high-speed steels. It maintains those properties at the high temperatures generated when cutting metals. Tungsten and molybdenum are interchangeable at an atomic level, and both promote resistance to tempering, which gives improved tool cutting performance at higher temperatures.
- Chromium. Chromium increases hardness, strength, and corrosion resistance. The strengthening effect of forming stable metal carbides at the grain boundaries and the strong increase in corrosion resistance made chromium an important alloying material for steel. Generally speaking, the concentration specified for most grades is approximately 4%, and this level appears to result in the best balance between hardness and toughness. Chromium plays an important role in the hardening mechanism and is considered irreplaceable. At higher temperatures, chromium contributes to increased strength, and it is ordinarily used for applications of this nature in conjunction with molybdenum.
- Molybdenum. When added to tool steel, molybdenum (about 0.50-8.00%) makes it more resistant to high temperatures. Molybdenum increases hardenability and strength, particularly at high temperatures, due to the high melting point of molybdenum. Molybdenum is unique in the extent to which it increases steel’s high-temperature tensile and creeps strengths. It retards the transformation of austenite to pearlite far more than the transformation of austenite to bainite; thus, bainite may be produced by continuous cooling of molybdenum-containing steels.
- Vanadium. Vanadium is generally added to steel to inhibit grain growth during heat treatment. Controlling grain growth improves the strength and toughness of hardened and tempered steels. The size of the grain determines the properties of the metal. For example, smaller grain size increases tensile strength and tends to increase ductility. Larger grain size is preferred for improved high-temperature creep properties. Vanadium is added to promote abrasion resistance and to produce hard and stable carbides, which are only partly soluble, and release little carbon into the matrix.
Properties of Molybdenum High-speed Steel – AISI M2
Material properties are intensive properties, which means they are independent of the amount of mass and may vary from place to place within the system at any moment. Materials science involves studying materials’ structure and relating them to their properties (mechanical, electrical, etc.). Once materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and how it has been processed into its final form.
Mechanical Properties of High-speed Steel – AISI M2
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial, and engineers must consider them.
Strength of High-speed Steel – AISI M2
In the mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. The strength of materials considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. The strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
The ultimate tensile strength of high-speed steel – AISI M2 depends on the heat treatment process, but it is about 1200 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the specimen preparation, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the specimen preparation, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
Yield Strength
The yield strength of high-speed steel – AISI M2 depends on the heat treatment process, but it is about 1000 MPa. Compressive yield strength is about 3250 Mpa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically. In contrast, the yield point is the point where nonlinear (elastic + plastic) deformation begins. Before the yield point, the material will deform elastically and return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behavior termed a yield point phenomenon. Yield strengths vary from 35 MPa for low-strength aluminum to greater than 1400 MPa for high-strength steel.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of high-speed steel – AISI M2 is 200 GPa.
Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to limiting stress, a body will be able to recover its dimensions on the removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position, and all the atoms are displaced the same amount and maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions, and no permanent deformation occurs. According to Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
The hardness of High-speed Steel – AISI M2
Rockwell hardness of high-speed steel – AISI M2 depends on the heat treatment process, but it is approximately 65 HRC.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to the Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position, and the major load is applied, then removed while maintaining the minor load. The difference between the penetration depth before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of High-speed Steel – AISI M2
Thermal properties of materials refer to the response of materials to changes in their temperature and to the application of heat. As a solid absorbs energy in the form of heat, its temperature rises and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are often critical in solids’ practical use.
Melting Point of High-speed Steel – AISI M2
The melting point of high-speed steel – AISI M2 steel is around 1430°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition where the solid and liquid can exist in equilibrium.
Thermal Conductivity of High-speed Steel – AISI M2
The thermal conductivity of high-speed steel – AISI M2 is 41 W/(m. K).
The heat transfer characteristics of solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It measures a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies to all matter, regardless of its state (solid, liquid, or gas). Therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature, and for vapors, it also depends upon pressure. In general:
Most materials are nearly homogeneous. Therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz). However, for an isotropic material, the thermal conductivity is independent of the transfer direction, kx = ky = kz = k.