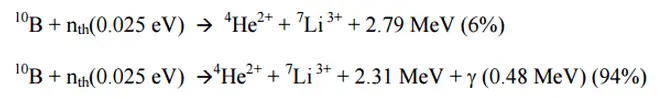Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B. Its (n,alpha) reaction cross-section for thermal neutrons is about 3840 barns (for 0.025 eV neutron). Isotope 11B has absorption cross-section for thermal neutrons about 0.005 barns (for 0.025 eV neutron). Most of (n,alpha) reactions of thermal neutrons are 10B(n,alpha)7Li reactions accompanied by 0.48 MeV gamma emission.
Moreover, isotope 10B has a high (n, alpha) reaction cross-section along the entire neutron energy spectrum. The cross-sections of most other elements become very small at high energies, as in the case of cadmium. The cross-section of 10B decreases monotonically with energy. For fast neutrons, its cross-section is on the order of barns.
Boron, as the neutron absorber, has another positive property. The reaction products (after a neutron absorption), helium and lithium, are stable isotopes. Therefore there are minimal problems with decay heating of control rods or burnable absorbers used in the reactor core.
On the other hand production of helium may lead to a significant increase in pressure (under rod cladding) when used as the absorbing material in control rods. Moreover, 10B is the principal source of radioactive tritium in the primary circuit of all PWRs (which use boric acid as a chemical shim) because reactions with neutrons can rarely lead to the formation of radioactive tritium via:
10B(n,2x alpha)3H threshold reaction (~1.2 MeV)
and
10B(n,alpha)7Li(n,n+alpha)3H threshold reaction (~3 MeV).
See also: Tritium
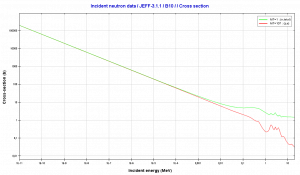
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library
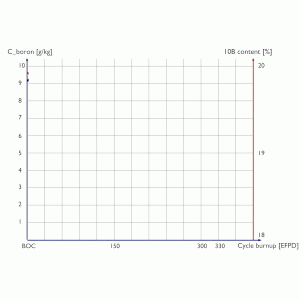
Boron letdown curve (chemical shim) and boron 10 depletion during a 12-month fuel cycle.
At the beginning of specific fuel cycle concentration of boric acid is highest. At the end of this cycle concentration of boric acid is almost zero and a reactor must be refueled, because there is no positive reactivity that can be inserted to compensate negative reactivity of fuel burnup (increase in reactor slagging).