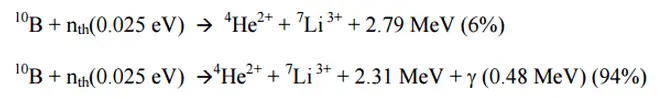Natural boron consists primarily of two stable isotopes, 11B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B. Its (n,alpha) reaction cross-section for thermal neutrons is about 3840 barns (for 0.025 eV neutron). Isotope 11B has absorption cross-section for thermal neutrons about 0.005 barns (for 0.025 eV neutron). Most of (n,alpha) reactions of thermal neutrons are 10B(n,alpha)7Li reactions accompanied by 0.48 MeV gamma emission.
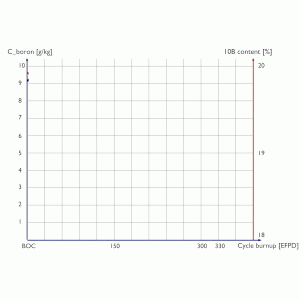
Since the isotope 10B has a significantly higher neutron cross-section, the 10B depletes much faster than 11B. Without adding fresh boron (19,9% of 10B) into the primary coolant system, the enrichment of 10B in boric acid continuously decreases. As a result, the enrichment of 10B at the end of the fuel cycle can be, for example, below 18% of 10B. This phenomenon must be considered in all the criticality calculations (e.g.,, shutdown margin calculations, estimated critical conditions, or general core depletion calculations).
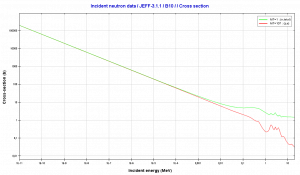
Source: JANIS (Java-based Nuclear Data Information Software); The JEFF-3.1.1 Nuclear Data Library