For heavy nuclides with an atomic number higher than 90, most fissile isotopes meet the fissile rule:
Fissile isotopes have 2 x Z – N = 43 ± 2 (example for 235U: 2 x 92 – 143 = 41)
where Z is the number of protons and N is the number of neutrons.
The distinction between Fissionable, Fissile, and Fertile
Fissile materials undergo fission reaction after absorption of the binding energy of the thermal neutron. They do not require additional kinetic energy for fission. If the neutron has higher kinetic energy, this energy will be transformed into the additional excitation energy of the compound nucleus. On the other hand, the binding energy released by the compound nucleus of (238U + n) after absorption of the thermal neutron is less than the critical energy, so the fission reaction cannot occur. The distinction is described in the following points.
- Fissile materials are a subset of fissionable materials.
- Fissionable material consists of isotopes that are capable of undergoing nuclear fission after capturing either fast neutron (high energy neutron – let say >1 MeV) or thermal neutron (low energy neutron – let say 0.025 eV). Typical fissionable materials: 238U, 240Pu, but also 235U, 233U, 239Pu, 241Pu
- Fissile material consists of fissionable isotopes capable of undergoing nuclear fission only after capturing a thermal neutron. 238U is not a fissile isotope because a thermal neutron cannot fission 238U. 238U does not also meet the alternative requirement to fissile materials. 238U cannot sustain a nuclear fission chain reaction because neutrons produced by the fission of 238U have lower energies than the original neutron (usually below the threshold energy of 1 MeV). Typical fissile materials: 235U, 233U, 239Pu, 241Pu.
- Fertile material consists of isotopes that are not fissionable by thermal neutrons but can be converted into fissile isotopes (after neutron absorption and subsequent nuclear decay). Typical fertile materials: 238U, 232Th.
See also: Neutron cross-section.
Comparison of cross-sections
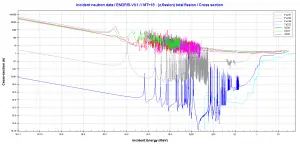
Source: JANIS (Java-based Nuclear Data Information Software)
http://www.oecd-nea.org/janis/
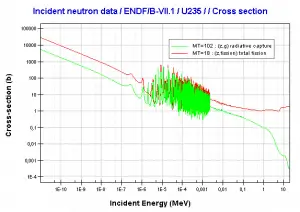
http://www.oecd-nea.org/janis/
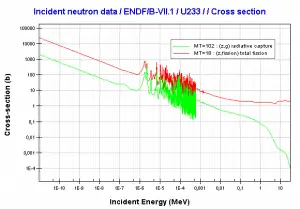
Source: JANIS (Java-based nuclear information software)
http://www.oecd-nea.org/janis/
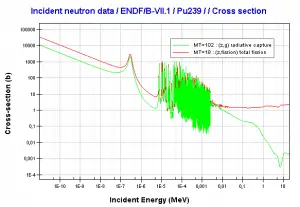
Source: JANIS (Java-based nuclear information software)
http://www.oecd-nea.org/janis/
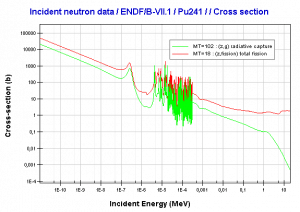
Source: JANIS (Java-based nuclear information software)
http://www.oecd-nea.org/janis/