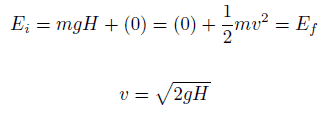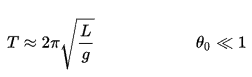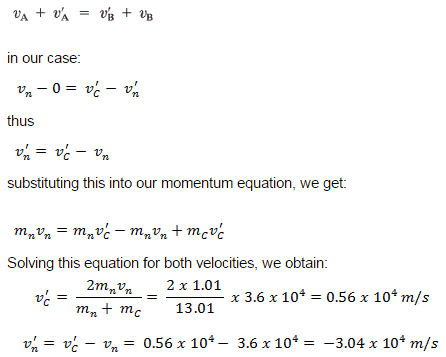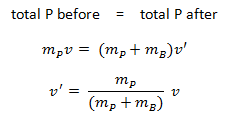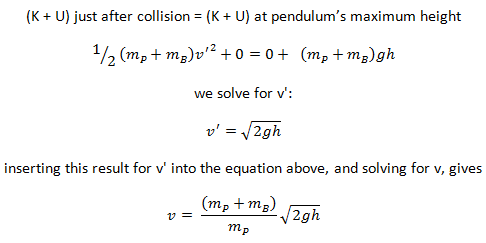Law of Conservation of Energy
The law of conservation of energy is one of the basic laws of physics, along with the conservation of mass and the conservation of momentum. The law of conservation of energy states that energy can change from one form into another, but it cannot be created or destroyed. Or the general definition is:
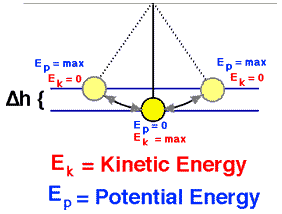
The total energy of an isolated system remains constant over time.
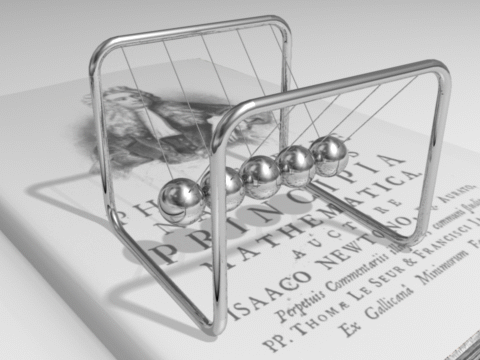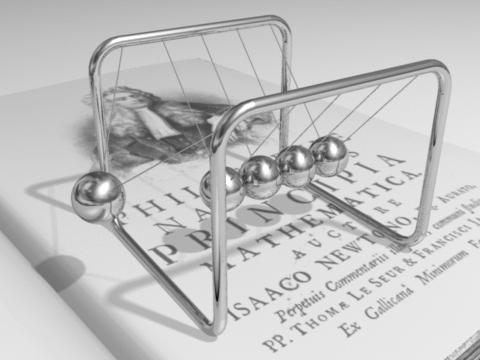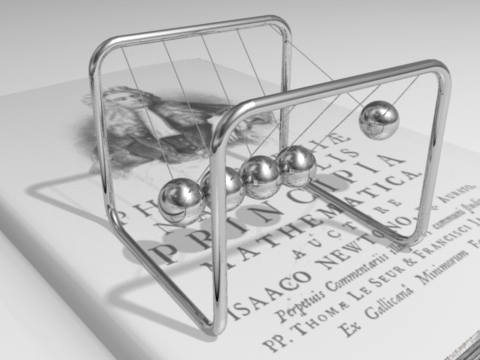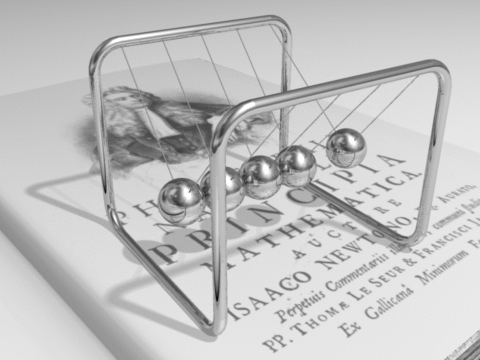
Energy can be defined as the capacity for doing work. It may exist in various forms and may be transformed from one type of energy to another in hundreds of ways.
For example, burning gasoline to power cars is an energy conversion process we rely on. The chemical energy in gasoline is converted to thermal energy, then converted to mechanical energy that makes the car move. The mechanical energy has been converted to kinetic energy. When we use the brakes to stop a car, that kinetic energy is converted by friction back to heat or thermal energy.
A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind, which produces work without the input of energy, cannot exist.
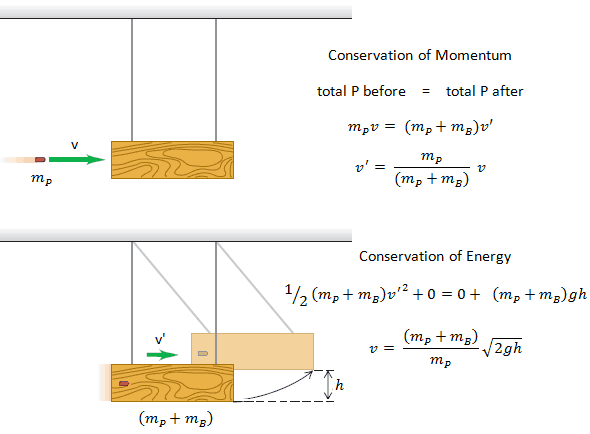
Example: Conservation of Mechanical Energy
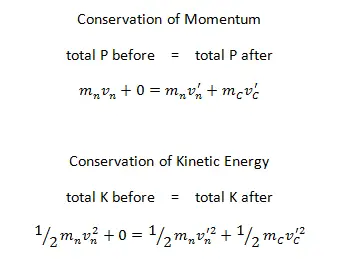
Example: Elastic Collisions
Example: Inelastic Collisions
Conservation of Energy in Chemical Reactions
Example: Combustion of Hydrogen
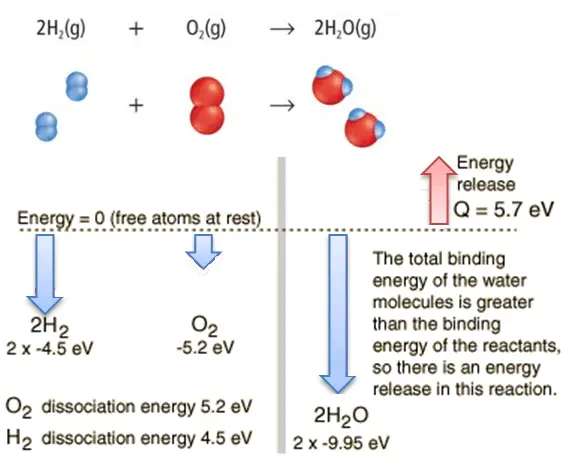
Consider the combustion of hydrogen in air. In a flame of pure hydrogen gas burning in the air, the hydrogen (H2) reacts with oxygen (O2) to form water (H2O) and releases energy.
Energetically, the process can be considered to require the energy to dissociate the H2 and O2, but then the bonding of the H2O returns the system to a bound state with negative potential. It is more negative than the bound states of the reactants, and the formation of the two water molecules is, therefore, an exothermic reaction, which releases 5.7 eV of energy.
2H2(g) + O2(g) → 2H2O(g)
The balance of energy before and after the reaction can be illustrated schematically with the state in which all atoms are free taken as the reference for energy.