Article Summary & FAQs
What is the law of conservation of matter?
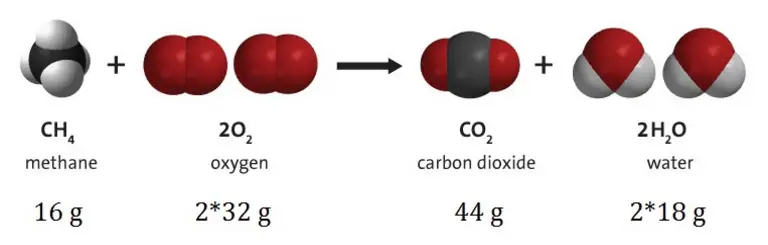
The law of conservation of matter is a general law in physics and chemistry applicable for any system closed to all transfers of matter and energy. According to this law, the mass of an object or collection of objects never changes over time, no matter how the constituent parts rearrange themselves. In other words, the mass can neither be created nor destroyed.
Key Facts
- The concept of mass conservation is widely used in many fields such as physics, chemistry, mechanics, and fluid dynamics.
- In chemistry, the calculation of the amount of reactant and products in a chemical reaction, or stoichiometry, is founded on the principle of conservation of mass.
- In fluid dynamics, this law is represented by the continuity equation, which states that all mass flow rates into a control volume are equal to all mass flow rates out of the control volume plus the rate of change of mass within the control volume.
- In the special theory of relativity, certain types of matter may be created or destroyed. Still, the mass and energy associated with such matter remain unchanged in quantity in all of these processes.
The Law of Conservation of Matter – Conservation of Mass
The law of conservation of matter or principle of matter conservation states that the mass of an object or collection of objects never changes over time, no matter how the constituent parts rearrange themselves.
The mass can neither be created nor destroyed.
The law requires that during any nuclear reaction, radioactive decay or chemical reaction in an isolated system, the total mass of the reactants or starting materials must be equal to the mass of the products.
Historically, already the ancient Greeks proposed the idea that the total amount of matter in the universe is constant. Mikhail Lomonosov first outlined the principle of conservation of mass in 1748. However, the law of conservation of matter (or the principle of mass/matter conservation) as a fundamental principle of physics was discovered by Antoine Lavoisier in the late 18th century. It was of great importance in progressing from alchemy to modern chemistry. Before this discovery, there were questions like:
- Why a piece of wood weighs less after afterburning?
- Can a matter or some of its part disappear?
In the case of burned wood, the problem was the measurement of the weight of released gases. Measurements of the weight of released gases were complicated because of the buoyancy effect of the Earth’s atmosphere on the weight of gases. Once understood, the conservation of matter was crucial in progress from alchemy to the modern natural science of chemistry.
The Law of Conservation of Matter in Special Relativity Theory
At the beginning of the 20th century, the notion of mass underwent a radical revision. The mass lost its absoluteness. One of the striking results of Einstein’s theory of relativity is that mass and energy are equivalent and convertible one into the other. Equivalence of the mass and energy is described by Einstein’s famous formula E = mc2. In other words, energy equals mass multiplied by the speed of light squared. Because the speed of light is very large, the formula implies that any small amount of matter contains a very large amount of energy. The mass of an object was seen as equivalent to energy, interconvertible with energy, and increasing significantly at exceedingly high speeds near that of light. The total energy was understood to comprise its rest mass and its increase of mass caused by increased kinetic energy.
In the special theory of relativity, certain types of matter may be created or destroyed. Still, the mass and energy associated with such matter remain unchanged in quantity in all of these processes. It was found the rest mass of an atomic nucleus is measurably smaller than the sum of the rest masses of its constituent protons, neutrons, and electrons. Mass was no longer considered unchangeable in the closed system. The difference is a measure of the nuclear binding energy which holds the nucleus together. According to the Einstein relationship (E = mc2), this binding energy is proportional to this mass difference, known as the mass defect.
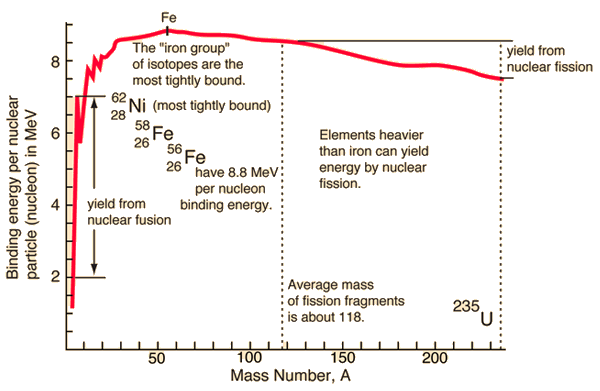
Source: hyperphysics.phy-astr.gsu.edu
During the nuclear splitting or nuclear fusion, some of the mass of the nucleus gets converted into huge amounts of energy. Thus this mass is removed from the total mass of the original particles, and the mass is missing in the resulting nucleus. The nuclear binding energies are enormous, and they are a million times greater than the electron binding energies of atoms.
Generally, in both chemical and nuclear reactions, some conversion between rest mass and energy occurs so that the products generally have smaller or greater mass than the reactants. Therefore the new conservation principle is the conservation of mass energy.
See also: Energy Release from Fission.
The Law of Conservation of Matter in Fluid Dynamics
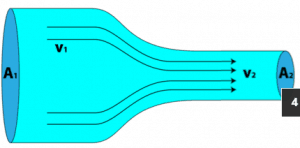
This principle is generally known as the conservation of matter principle. It states that the mass of an object or collection of objects never changes over time, no matter how the constituent parts rearrange themselves. This principle can be used in the analysis of flowing fluids. Conservation of mass in fluid dynamics states that all mass flow rates into a control volume are equal to all mass flow rates out of the control volume plus the rate of mass change within the control volume. This principle is expressed mathematically by the following equation:
ṁin = ṁout +∆m⁄∆t
Mass entering per unit time = mass leaving per unit time + Increase of mass in the control volume per unit time
This equation describes nonsteady-state flow. Nonsteady-state flow refers to the condition where the fluid properties at any single point in the system may change over time. Steady-state flow refers to the condition where the fluid properties (temperature, pressure, and velocity) at any single point in the system do not change over time. But one of the most significant constant properties in a steady-state flow system is the system mass flow rate. This means that there is no accumulation of mass within any component in the system.
See also: Continuity Equation
Continuity Equation
The continuity equation is simply a mathematical expression of the principle of conservation of mass. For a control volume with a single inlet and a single outlet, the principle of conservation of mass states that, for steady-state flow, the mass flow rate into the volume must equal the mass flow rate out.
ṁin = ṁout
Mass entering per unit time = mass leaving per unit time
This equation is called the continuity equation for steady one-dimensional flow. The net mass flow must be zero for a steady flow through a control volume with many inlets and outlets, where negative inflows and outflows are positive.
This principle can be applied to a stream tube such as that shown above. No fluid flows across the boundary made by the streamlines, so mass only enters and leaves through the two ends of this stream tube section.
When a fluid is in motion, it must move in such a way that mass is conserved. To see how mass conservation places restrictions on the velocity field, consider the steady flow of fluid through a duct (that is, the inlet and outlet flows do not vary with time).
Differential Form of Continuity Equation
A general continuity equation can also be written in a differential form:
∂⍴⁄∂t + ∇ . (⍴ ͞v) = σ
where
- ∇ . is divergence,
- ρ is the density of quantity q,
- ⍴ ͞v is the flux of quantity q,
- σ is the generation of q per unit volume per unit time. Terms that generate (σ > 0) or remove (σ < 0) q are referred to as a “sources” and “sinks” respectively. If q is a conserved quantity (such as energy), σ is equal to 0.