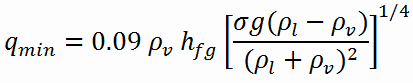The fact that a water drop is long-lived when deposited on metal much hotter than the boiling temperature of the water was first reported by Hermann Boerhaave in 1732. It was not investigated extensively until 1756 when a German doctor Johann Gottlob Leidenfrost published “A Tract About Some Qualities of Common Water.”
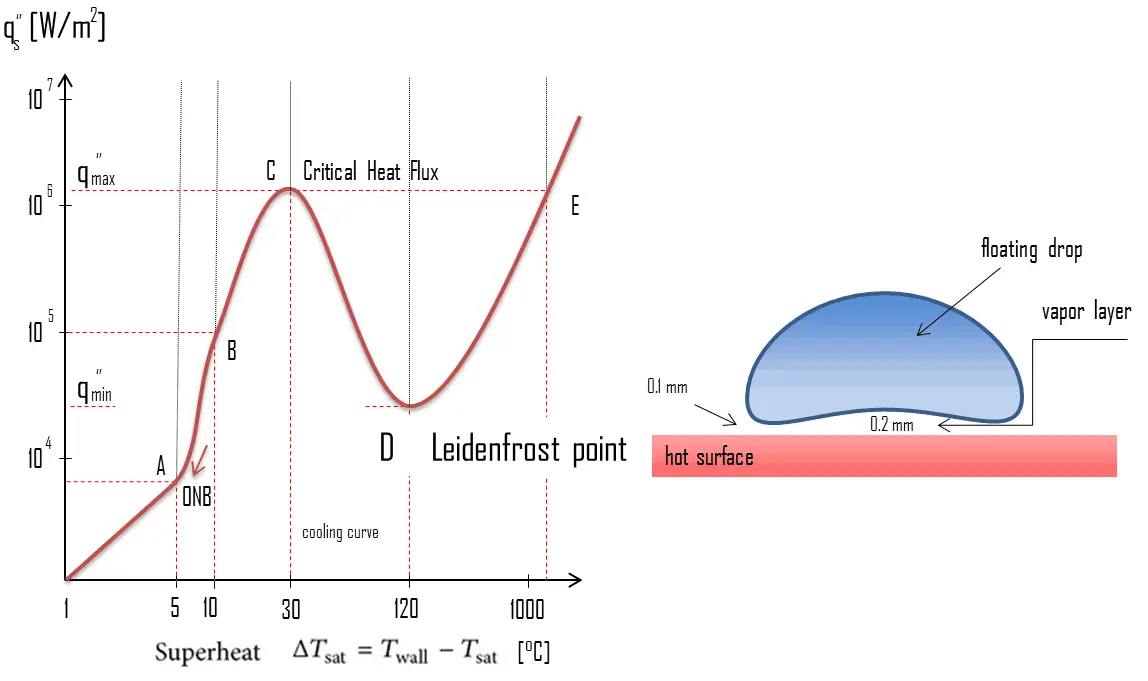
This effect can be commonly demonstrated during cooking when one sprinkles drops of water in a pan to gauge its temperature: if the pan’s temperature is at or above the Leidenfrost point, the water skitters across the pan and takes longer to evaporate than in a pan below the temperature of the Leidenfrost point (but still above boiling temperature). The Leidenfrost point, which corresponds to the minimal heat flux, is of practical interest since it represents the lower limit for the heat flux in the film boiling regime. If the heat flux drops below this minimum, the film will collapse, causing the surface to cool and nucleate boiling to be reestablished. The Leidenfrost effect is also responsible for the ability of liquid nitrogen to skitter across floors.
Minimum Heat Flux – Leidenfrost Point
 The Leidenfrost point, which corresponds to the minimal heat flux, is of practical interest since it represents the lower limit for the heat flux in the film boiling regime. If the heat flux drops below this minimum, the film will collapse, causing the surface to cool and nucleate boiling to be reestablished. Therefore, return to nucleate boiling (RNB) occurs at this point. The terms quenching, minimum heat flux, return to nucleate boiling, departure from film boiling, film boiling collapse, and Leidenfrost point have been used interchangeably to refer to various forms of rewetting, but they are not exactly synonymous.
The Leidenfrost point, which corresponds to the minimal heat flux, is of practical interest since it represents the lower limit for the heat flux in the film boiling regime. If the heat flux drops below this minimum, the film will collapse, causing the surface to cool and nucleate boiling to be reestablished. Therefore, return to nucleate boiling (RNB) occurs at this point. The terms quenching, minimum heat flux, return to nucleate boiling, departure from film boiling, film boiling collapse, and Leidenfrost point have been used interchangeably to refer to various forms of rewetting, but they are not exactly synonymous.
Using the stability theory, Zuber derived the following expression for the minimum heat flux (and corresponding Leidenfrost point) for a large horizontal plate:
where
- qmin – minimal heat flux [W/m2]
- hfg – enthalpy of vaporization, J/kg
- g – gravitational acceleration m/s2
- ρl — density of the liquid kg/m3
- ρv — density of vapor kg/m3
- σ — surface tension-liquid-vapor interface N/m