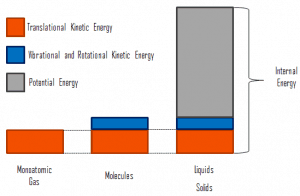
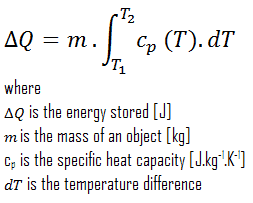 In thermodynamics, internal energy (also called thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, and it depends on the size of the system or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the system’s potential energy. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly. Still, techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
In thermodynamics, internal energy (also called thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, and it depends on the size of the system or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the system’s potential energy. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly. Still, techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
Heat Absorption
In addition, energy can be stored in the chemical bonds between the atoms that make up the molecules. This energy storage on the atomic level includes energy associated with electron orbital states, nuclear spin, and binding forces in the nucleus.

Thermal energy can also be very effectively absorbed and stored. Nowadays, the situation in energy markets is different. The increase in the prices of conventional energy sources and environmental awareness has led to increased renewable energies and energy efficiency. Thermal energy storage forms a key component of a power plant to improve its dispatchability, especially for concentrating solar power plants (CSP). Thermal energy storage (TES) is achieved with widely differing technologies. There are three methods used and still being investigated to store thermal energy.
- Sensible Heat Storage (SHS)
- Latent Heat Storage (LHS)
- Thermo-chemical Storage
Absorption in Sensible Heat
The most direct way is the storage of sensible heat. Sensible heat storage is based on raising the temperature of a liquid or solid to store heat and releasing it with a decrease in temperature when required. The volumes needed to store energy on the scale that the world needs are extremely large. Materials used in sensible heat storage must have a high heat capacity and high boiling or melting point. Although this heat storage method is currently less efficient for heat storage, it is least complicated compared with latent or chemical heat, and it is inexpensive.
From a thermodynamics point of view, the storage of sensible heat is based on the increase of enthalpy of the material in the store, either a liquid or a solid, in most cases. The sensible effect is a temperature change. Heat stored can be obtained by the equation:
Absorption in Latent Heat
A common approach to thermal energy storage is to use phase change materials (PCMs). These materials store heat when they undergo a phase change, for example, from solid to liquid, from liquid to gas, or from solid to solid (change of one crystalline form into another without a physical phase change).
The phase change “solid-to-liquid” is the most used, but solid-to-solid change is of interest. These materials can be used as an effective way of storing thermal energy (solar energy, off-peak electricity, industrial waste heat). In comparison to sensible heat storage systems, latent heat storage has the advantages of high storage density (due to high latent heat of fusion) and the isothermal nature of the storage process. The heat of fusion or the heat of evaporation is much greater than the specific heat capacity. The comparison between latent heat storage and sensible heat storage shows that latent heat storage densities are typically 5 to 10 times higher.
In general, latent heat effects associated with the phase change are significant. Latent heat, also known as the enthalpy of vaporization (liquid-to-vapor phase change) or enthalpy of fusion (solid-to-liquid phase change), is the amount of heat added to or removed from a substance to produce a phase change. This energy breaks down the intermolecular attractive forces and must provide the energy necessary to expand the substance (the pΔV work). When latent heat is added, no temperature change occurs.
Absorption in Chemical Energy
One of three possible approaches to thermal energy storage is reversible thermo-chemical reactions. The most important advantage of the thermo-chemical storage method is that the enthalpy of reaction is considerably larger than the specific heat or the heat of fusion. Therefore the storage density is much better. In chemical reactions, energy is stored in the chemical bonds between the atoms that make up the molecules. Energy storage on the atomic level includes energy associated with electron orbital states. Whether a chemical reaction absorbs or releases energy, there is no overall change in the amount of energy during the reaction. That’s because of the law of conservation of energy, which states that:
Energy cannot be created or destroyed. Energy may change form during a chemical reaction.
One example of an experimental storage system based on chemical reaction energy is salt hydrate technology. The system is especially advantageous for seasonal thermal energy storage. The system uses the reaction energy created when salts are hydrated or dehydrated. It works by storing heat in a container containing 50% sodium hydroxide (NaOH) solution. Heat (e.g., using a solar collector) is stored by evaporating the water in an endothermic reaction. When water is added again, heat is released in an exothermic reaction at 50 °C. Current systems operate at 60% efficiency.
Radiant Heat Absorption
 In preceding chapters, we have discussed convection and conduction, which require the presence of matter as a medium to carry the heat from the hotter to the colder region. But the third type of heat transfer, radiation heat transfer, occurs without any medium at all. In general, the radiation heat transfer from one surface to another is the radiation leaving the first surface for the other minus that arriving from the second surface. Radiation heat transfer is mediated by electromagnetic radiation, known as thermal radiation, that arises due to the temperature of a body.
In preceding chapters, we have discussed convection and conduction, which require the presence of matter as a medium to carry the heat from the hotter to the colder region. But the third type of heat transfer, radiation heat transfer, occurs without any medium at all. In general, the radiation heat transfer from one surface to another is the radiation leaving the first surface for the other minus that arriving from the second surface. Radiation heat transfer is mediated by electromagnetic radiation, known as thermal radiation, that arises due to the temperature of a body.
Another important radiation property of a surface is its absorptivity, α, which is the fraction of the radiation energy incident on a surface absorbed by the surface. Like emissivity, value of absorptivity is in the range 0 < α < 1.
From its definition, a blackbody, which is an idealized physical body, absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. That is, a blackbody is a perfect absorber. Since, for real objects, the absorptivity is less than unity, a real object can not absorb all incident light. The incomplete absorption can be due to some of the incident light being transmitted through the body or to some of it being reflected at the body’s surface.
In general, the absorptivity and the emissivity are interconnected by the Kirchhoff’s Law of thermal radiation, which states:
For an arbitrary body emitting and absorbing thermal radiation in thermodynamic equilibrium, the emissivity is equal to the absorptivity.
emissivity ε = absorptivity α
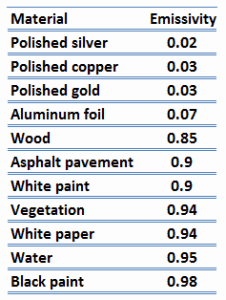
Note that visible radiation occupies a very narrow band of the spectrum from 400 to 760 nm. We cannot judge the blackness of a surface based on visual observations. For example, consider a white paper that reflects visible light and thus appears white. On the other hand, it is essentially black for infrared radiation (absorptivity α = 0.94) since they strongly absorb long-wavelength radiation.