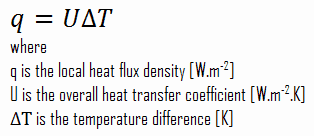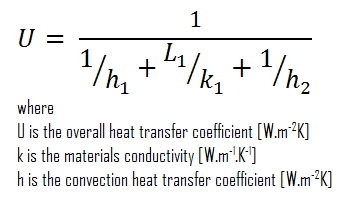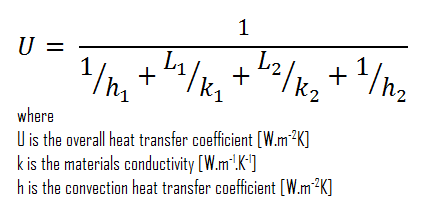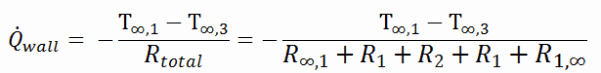A very important source of heat loss from a house is through the roof and attic. Attic insulation is a thermally insulated, protective interior cladding procedure involving the use of glass or rock wool, polyurethane foam, or phenolic foam. It must be noted that there is a difference between insulating a pitched roof and a flat roof, and there is a difference between cold or warm loft insulation. Cold roof insulation requires insulation at the joist level to stop heat from escaping through the unused roof space. A warm roof is insulated between and under the roof’s rafters.
A very important source of heat loss from a house is through the roof and attic. Attic insulation is a thermally insulated, protective interior cladding procedure involving the use of glass or rock wool, polyurethane foam, or phenolic foam. It must be noted that there is a difference between insulating a pitched roof and a flat roof, and there is a difference between cold or warm loft insulation. Cold roof insulation requires insulation at the joist level to stop heat from escaping through the unused roof space. A warm roof is insulated between and under the roof’s rafters.
The purpose of roof insulation is to reduce the overall heat transfer coefficient by adding materials with low thermal conductivity. Roof and attic insulation in buildings is an important factor in achieving thermal comfort for occupants. Roof insulation, as well as other types of insulation, reduces unwanted heat loss and also reduce unwanted heat gain. They can significantly decrease the energy demands of heating and cooling systems. It must be added that no material can completely prevent heat losses. Heat losses can only be minimized.
Blown-In and Loose-Fill Insulation
Loose-fill materials can be blown into attics and finished wall cavities. For existing buildings that were not built with insulated cavities, fibrous material such as cellulose insulation or glass wool is blown into the cavity through suitably drilled holes until it fills the entire wall space. Loose-fill insulation consists of small particles of fiber, foam, or other materials. The most common types of materials used for loose-fill insulation include cellulose, glass wool, and rock wool.
- Cellulose insulation is made from recycled paper products, primarily newspapers, and has a very high recycled material content.
- Glass wool (originally known also as fiberglass) is an insulating material made from fibers of glass arranged using a binder into a texture similar to wool.
- Stone wool, also known as rock wool, is based on natural minerals present in large quantities throughout the earth, e.g., volcanic rock, typically basalt or dolomite.
These small particles made from these materials form an insulation material that can conform to any space without disturbing structures or finishes. One of the methods is Wet-spray cellulose insulation. This type of insulation is similar to loose-fill insulation but is applied with a small quantity of water to help the cellulose bind to the inside of open wall cavities.
Spray Foam Insulation
Spray foam insulation is a type of insulation sprayed in place through a gun. Spray foam insulation can be blown into walls, onto concrete slabs, on attic surfaces, or under floors to insulate and reduce air leakage. Spray foam can fill even the smallest cavities, creating an effective air barrier. After spraying in place, foam usually expands up to 30-60 times its liquid volume. It provides excellent resistance to air infiltration (unlike batts and blankets, which can leave bypasses and air pockets and are superior to some types of loose-fill). On the other hand, the cost of spray foam insulation can be higher compared to traditional insulation, and most foams, except for cementitious foams, release toxic fumes when they burn.
There are two types of spray foam insulation:
- Closed-cell foam. Closed-cell foams are better insulators, and their high-density cells are closed and filled with a gas that helps the foam expand to fill the spaces around it. Closed-cell foam is very strong and structurally reinforces the insulated surface.
- Open-cell foam. Open-cell foam cells are not as dense and are filled with air, which gives the insulation a spongy texture. Open-cell foam is porous, allowing water vapor and liquid water to penetrate the insulation. On the other hand, open-cell foams will allow structural wood to breathe, and they are about twice effective as a sound barrier.
Available foam insulation materials include:
- Cementitious
- Phenolic
- Polyisocyanurate
- Polyurethane.
Most are typically made with polyurethane or isocyanate. Cementitious foams are similar and can be applied in a similar manner but do not expand. These foams have higher fire resistance in comparison to polyurethane or isocyanate foams.
Insulation Materials
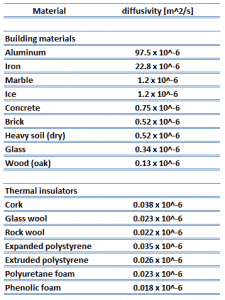 As was written, thermal insulation is based on the use of substances with very low thermal conductivity. These materials are known as insulation materials. Common insulation materials are wool, fiberglass, rock wool, polystyrene, polyurethane, goose feather, etc. Therefore, these materials are very poor conductors of heat and are good thermal insulators.
As was written, thermal insulation is based on the use of substances with very low thermal conductivity. These materials are known as insulation materials. Common insulation materials are wool, fiberglass, rock wool, polystyrene, polyurethane, goose feather, etc. Therefore, these materials are very poor conductors of heat and are good thermal insulators.
It must be added that thermal insulation is primarily based on the very low thermal conductivity of gases. Gases possess poor thermal conduction properties compared to liquids and solids and thus make a good insulation material if they can be trapped (e.g., in a foam-like structure). Air and other gases are generally good insulators. But the main benefit is in the absence of convection. Therefore, many insulation materials (e.g., polystyrene) function simply by having many gas-filled pockets, which prevent large-scale convection. In all types of thermal insulation, evacuation of the air in the void space will further reduce the overall thermal conductivity of the insulator.
Alternation of gas pocket and solid material causes heat to transfer through many interfaces, causing a rapid decrease in heat transfer coefficient.
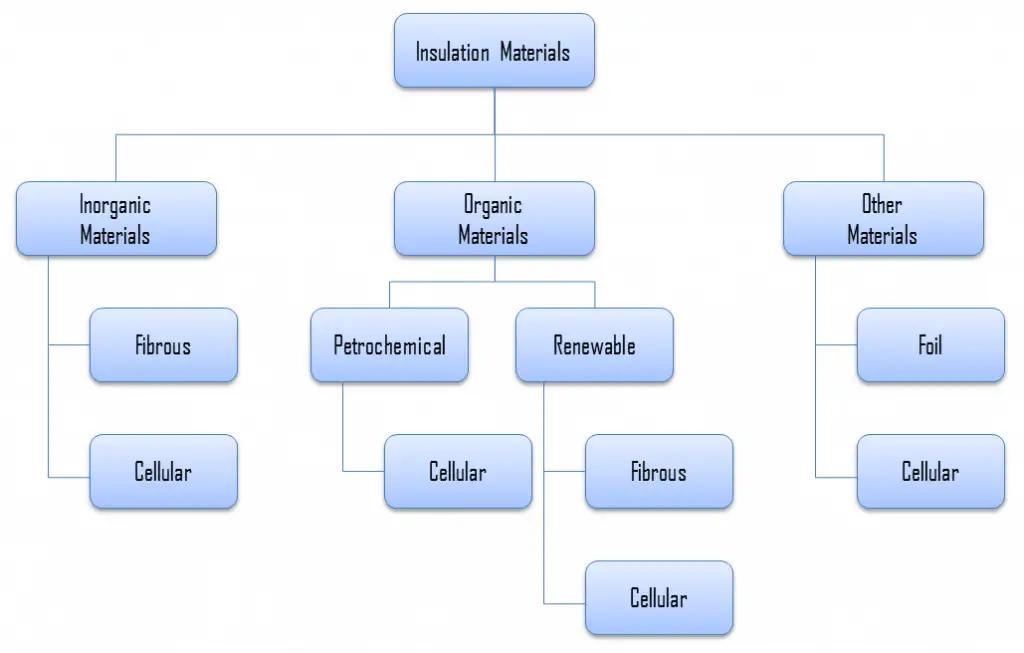
For insulation materials, three general categories can be defined. These categories are based on the chemical composition of the base material from which the insulating material is produced.
In further reading, there is a brief description of these types of insulation materials.
Inorganic Insulation Materials
As can be seen from the figure, inorganic materials can be classified accordingly:
- Fibrous materials
- Cellular materials
- Calcium silicate
- Cellular glass
Organic Insulation Materials
The organic insulation materials treated in this section are all derived from a petrochemical or renewable feedstock (bio-based). Almost all of the petrochemical insulation materials are in the form of polymers. As can be seen from the figure, all petrochemical insulation materials are cellular, and material is cellular when the material’s structure consists of pores or cells. On the other hand, many plants contain fibers for their strength. Therefore almost all the bio-based insulation materials are fibrous (except expanded cork, which is cellular).
Organic insulation materials can be classified accordingly:
- Petrochemical materials (oil/coal-derived)
- Renewable materials (plant/animal-derived)
- Cellulose
- Cork
- Woodfibre
- Hemp fiber
- Flax wool
- Sheep’s wool
- Cotton insulation
Other Insulation Materials
Example of Insulation – Glass Wool
 Glass wool (originally known also as fiberglass) is an insulating material made from fibers of glass arranged using a binder into a texture similar to wool. Glass wool and stone wool is produced from mineral fibers and are therefore often referred to as ‘mineral wools.’ Mineral wool is a general name for fiber materials formed by spinning or drawing molten minerals. Glass wool is a furnace product of molten glass at a temperature of about 1450 °C. From the melted glass, fibers are spun. This process is based on spinning molten glass in high-speed spinning heads, somewhat like the process used to produce cotton candy. A binding agent is injected during the spinning of the glass fibers. Glass wool is then produced in rolls or slabs, with different thermal and mechanical properties. It may also be produced as a material that can be sprayed or applied in place on the surface to be insulated.
Glass wool (originally known also as fiberglass) is an insulating material made from fibers of glass arranged using a binder into a texture similar to wool. Glass wool and stone wool is produced from mineral fibers and are therefore often referred to as ‘mineral wools.’ Mineral wool is a general name for fiber materials formed by spinning or drawing molten minerals. Glass wool is a furnace product of molten glass at a temperature of about 1450 °C. From the melted glass, fibers are spun. This process is based on spinning molten glass in high-speed spinning heads, somewhat like the process used to produce cotton candy. A binding agent is injected during the spinning of the glass fibers. Glass wool is then produced in rolls or slabs, with different thermal and mechanical properties. It may also be produced as a material that can be sprayed or applied in place on the surface to be insulated.
Applications of glass wool include structural insulation, pipe insulation, filtration, and soundproofing. Glass wool is a versatile material that can be used for the insulation of walls, roofs, and floors. It can be a loose-fill material, blown into attics, or, together with an active binder sprayed on the underside of structures. During the installation of the glass wool, it should be kept dry at all times since an increase in the moisture content causes a significant increase in thermal conductivity.
Example – Heat Loss through a Wall
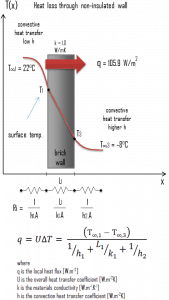 A major source of heat loss from a house is through walls. Calculate the rate of heat flux through a wall 3 m x 10 m in the area (A = 30 m2). The wall is 15 cm thick (L1), and it is made of bricks with thermal conductivity of k1 = 1.0 W/m.K (poor thermal insulator). Assume that the indoor and the outdoor temperatures are 22°C and -8°C, and the convection heat transfer coefficients on the inner and the outer sides are h1 = 10 W/m2K and h2 = 30 W/m2K, respectively. Note that these convection coefficients strongly depend especially on ambient and interior conditions (wind, humidity, etc.).
A major source of heat loss from a house is through walls. Calculate the rate of heat flux through a wall 3 m x 10 m in the area (A = 30 m2). The wall is 15 cm thick (L1), and it is made of bricks with thermal conductivity of k1 = 1.0 W/m.K (poor thermal insulator). Assume that the indoor and the outdoor temperatures are 22°C and -8°C, and the convection heat transfer coefficients on the inner and the outer sides are h1 = 10 W/m2K and h2 = 30 W/m2K, respectively. Note that these convection coefficients strongly depend especially on ambient and interior conditions (wind, humidity, etc.).
- Calculate the heat flux (heat loss) through this non-insulated wall.
- Now assume thermal insulation on the outer side of this wall. Use glass wool insulation 10 cm thick (L2) with the thermal conductivity of k2 = 0.023 W/m.K and calculate the heat flux (heat loss) through this composite wall.
Solution:
Many heat transfer processes involve composite systems and even involve a combination of conduction and convection. It is often convenient to work with an overall heat transfer coefficient, known as a U-factor with these composite systems. The U-factor is defined by an expression analogous to Newton’s law of cooling:
The overall heat transfer coefficient is related to the total thermal resistance and depends on the geometry of the problem.
- bare wall
Assuming one-dimensional heat transfer through the plane wall and disregarding radiation, the overall heat transfer coefficient can be calculated as:
The overall heat transfer coefficient is then:
U = 1 / (1/10 + 0.15/1 + 1/30) = 3.53 W/m2K
The heat flux can be then calculated simply as:
q = 3.53 [W/m2K] x 30 [K] = 105.9 W/m2
The total heat loss through this wall will be:
qloss = q . A = 105.9 [W/m2] x 30 [m2] = 3177W
- composite wall with thermal insulation
Assuming one-dimensional heat transfer through the plane composite wall, no thermal contact resistance, and disregarding radiation, the overall heat transfer coefficient can be calculated as:
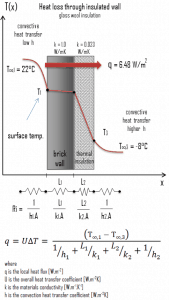 The overall heat transfer coefficient is then:
The overall heat transfer coefficient is then:
U = 1 / (1/10 + 0.15/1 + 0.1/0.023 + 1/30) = 0.216 W/m2K
The heat flux can be then calculated simply as:
q = 0.216 [W/m2K] x 30 [K] = 6.48 W/m2
The total heat loss through this wall will be:
qloss = q . A = 6.48 [W/m2] x 30 [m2] = 194 W
As can be seen, adding a thermal insulator causes a significant decrease in heat losses. It must be added that adding the next layer of the thermal insulator does not cause such high savings. This can be better seen from the thermal resistance method, which can be used to calculate the heat transfer through composite walls. The rate of steady heat transfer between two surfaces is equal to the temperature difference divided by the total thermal resistance between those two surfaces.