Article Summary & FAQs
What is radiation heat transfer?
Radiation heat transfer is mediated by electromagnetic radiation, known as thermal radiation, that arises due to the temperature of a body. Any material with a temperature above absolute zero gives off some radiant energy.
Key Facts
Heat is the amount of energy flowing spontaneously from one body to another due to its temperature difference. Heat is a form of energy, but it is energy in transit.
Heat transfer is usually classified into various mechanisms, such as:
-
- Heat Conduction. Heat conduction, also called diffusion, occurs within or between two bodies in contact. It is the direct microscopic exchange of kinetic energy of particles through the boundary between two systems.
- Heat Convection. Heat convection depends on the mass movement from one region of space to another. Heat convection occurs when the bulk flow (gas or liquid) carries heat along with the flow of matter in the fluid.
- Thermal Radiation. Radiation is heat transfer by electromagnetic radiation, such as sunshine, with no need to be present in the space between bodies.
Radiation heat transfer occurs without any medium at all.
Blackbody radiation is also called thermal radiation, cavity radiation, complete radiation, or temperature radiation. The following laws are associated with blackbody radiation:
- Kirchhoff’s law. This law gives the relationship between the emissivity and absorptivity of an object.
- Planck’s law. This law describes the spectrum of blackbody radiation, which depends only on the object’s temperature.
- Wien’s displacement law. This law determines the most likely frequency of the emitted radiation.
- Stefan–Boltzmann law. This law gives the radiant intensity.
In preceding chapters, we have discussed convection and conduction, which require the presence of matter as a medium to carry the heat from the hotter to the colder region. But the third type of heat transfer, radiation heat transfer, occurs without any medium at all. In general, the radiation heat transfer from one surface to another is the radiation leaving the first surface for the other minus that arriving from the second surface.
Source: hyperphysics.phy-astr.gsu.edu
Radiation heat transfer is mediated by electromagnetic radiation, known as thermal radiation, that arises due to the temperature of a body. Any material with a temperature above absolute zero gives off some radiant energy. Most energy of this type is in the infra-red region of the electromagnetic spectrum, although some of it is in the visible region. One of the most important examples of radiation heat transfer is the Earth’s absorption of solar radiation, followed by its outgoing thermal radiation. These processes determine the temperature and climate of the Earth.
Thermal Radiation
Thermal radiation is electromagnetic radiation in the infra-red region of the electromagnetic spectrum, although some of it is in the visible region. The term thermal radiation is frequently used to distinguish this form of electromagnetic radiation from other forms, such as radio waves, x-rays, or gamma rays. It is generated by the thermal motion of charged particles in matter, and therefore, any material with a temperature above absolute zero gives off some radiant energy. Thermal radiation does not require any medium for energy transfer. Energy transfer by radiation is the fastest (at the speed of light), and it suffers no attenuation in a vacuum.
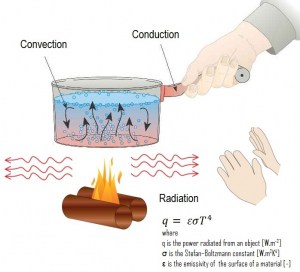 In contrast to heat transfer by conduction or convection, which occurs in the direction of decreasing temperature, thermal radiation heat transfer can occur between two bodies separated by a medium colder than both bodies. For example, solar radiation reaches the surface of the earth after passing through cold layers of the atmosphere at high altitudes.
In contrast to heat transfer by conduction or convection, which occurs in the direction of decreasing temperature, thermal radiation heat transfer can occur between two bodies separated by a medium colder than both bodies. For example, solar radiation reaches the surface of the earth after passing through cold layers of the atmosphere at high altitudes.
Stefan–Boltzmann Law
Radiation heat transfer rate, q [W/m2], from a body (e.g., a black body) to its surroundings is proportional to the fourth power of the absolute temperature. It can be expressed by the following equation:
q = εσT4
where σ is a fundamental physical constant called the Stefan–Boltzmann constant, equal to 5.6697×10-8 W/m2K4. The Stefan–Boltzmann constant is named after Josef Stefan (who discovered the Stefan-Boltzman law experimentally in 1879) and Ludwig Boltzmann (who derived it theoretically soon after). As can be seen, radiation heat transfer is important at very high temperatures and in a vacuum.
As was written, the Stefan–Boltzmann law gives the radiant intensity of a single object. But using the Stefan–Boltzmann law, we can also determine the radiation heat transfer between two objects. Two bodies that radiate toward each other have a net heat flux between them. The net flow rate of heat between them is given by:
Q = εσA1-2(T41 −T42) [J/s]
q = εσ(T41 −T42) [J/m2s]
The area factor A1-2 is the area viewed by body 2 of body 1 and can become fairly difficult to calculate.
Blackbody Radiation
It is known that the amount of radiation energy emitted from a surface at a given wavelength depends on the material of the body and the condition of its surface, and the surface temperature. Therefore, various materials emit different amounts of radiant energy even when they are at the same temperature. A body that emits the maximum amount of heat for its absolute temperature is called a blackbody.
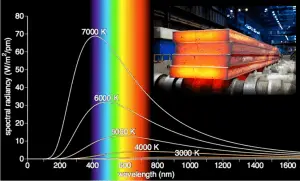 A blackbody is an idealized physical body that has specific properties. By definition, a black body in thermal equilibrium emissivity of ε = 1.0. Real objects do not radiate as much heat as a perfect black body, and they radiate less heat than a black body and therefore are called gray bodies.
A blackbody is an idealized physical body that has specific properties. By definition, a black body in thermal equilibrium emissivity of ε = 1.0. Real objects do not radiate as much heat as a perfect black body, and they radiate less heat than a black body and therefore are called gray bodies.
The surface of a blackbody emits thermal radiation at the rate of approximately 448 watts per square meter at room temperature (25 °C, 298.15 K). Real objects with emissivities less than 1.0 (e.g., copper wire) emit radiation at correspondingly lower rates (e.g., 448 x 0.03 = 13.4 W/m2). Emissivity plays an important role in heat transfer problems. For example, solar heat collectors incorporate selective surfaces with very low emissivities. These collectors waste very little solar energy through the emission of thermal radiation.
Since the absorptivity and the emissivity are interconnected by Kirchhoff’s Law of thermal radiation, a blackbody is also a perfect absorber of electromagnetic radiation.
Kirchhoff’s Law of thermal radiation:
For an arbitrary body emitting and absorbing thermal radiation in thermodynamic equilibrium, the emissivity is equal to the absorptivity.
emissivity ε = absorptivity α
A blackbody absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence, and its absorptivity is therefore equal to unity, which is also the highest possible value. A blackbody is a perfect absorber (and a perfect emitter).
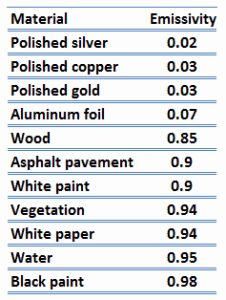
Note that visible radiation occupies a very narrow band of the spectrum from 400 to 760 nm. We cannot make any judgments about the blackness of a surface based on visual observations. For example, consider a white paper that reflects visible light and thus appears white. On the other hand, it is essentially black for infrared radiation (absorptivity α = 0.94) since they strongly absorb long-wavelength radiation.
See also: Ultraviolet Catastrophe
Blackbody Emissive Power
The blackbody emissive power, Eb [W/m2], from a blackbody to its surroundings is proportional to the fourth power of the absolute temperature and can be expressed by the following equation:
Eb = σT4
where σ is a fundamental physical constant called the Stefan–Boltzmann constant, equal to 5.6697×10-8 W/m2K4, and T is the absolute temperature of the surface in K.
The term blackbody was introduced by German physicist Gustav Kirchhoff in 1860. Blackbody radiation is also called thermal radiation, cavity radiation, complete radiation, or temperature radiation. Three following laws are associated with blackbody radiation:
- Kirchhoff’s law. This law gives the relationship between the emissivity and absorptivity of an object.
- Planck’s law. This law describes the spectrum of blackbody radiation, which depends only on the object’s temperature.
- Wien’s displacement law. This law determines the most likely frequency of the emitted radiation.
- Stefan–Boltzmann law. This law gives the radiant intensity.
All bodies above absolute zero temperature radiate some heat, and the sun and earth both radiate heat toward each other. This seems to violate the Second Law of Thermodynamics, which states that heat cannot spontaneously flow from cold system to hot system without external work being performed on the system. The paradox is resolved because each body must be in direct sight of the other to receive radiation from it. Therefore, whenever the cool body is radiating heat to the hot body, the hot body must also be radiating heat to the cool body. Moreover, the hot body will radiate more energy than the cold body. The case of different emissivities is solved by Kirchhoff’s Law of thermal radiation, which states that objects with low emissivity also have low absorptivity. As a result, heat cannot spontaneously flow from cold system to hot system, and the second law is still satisfied.