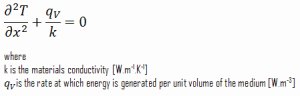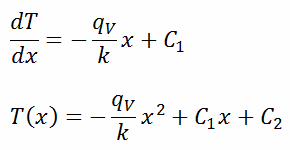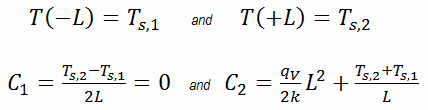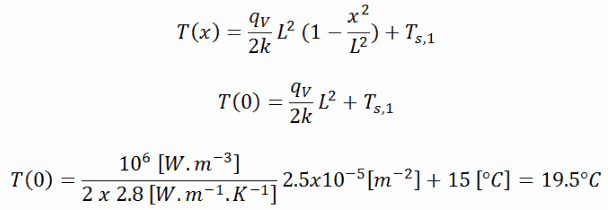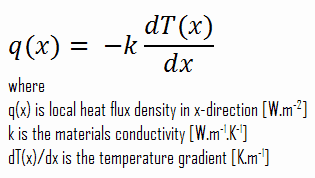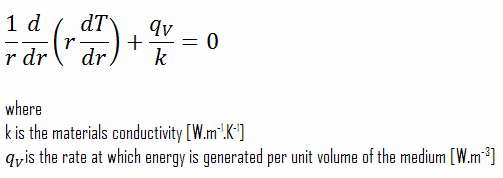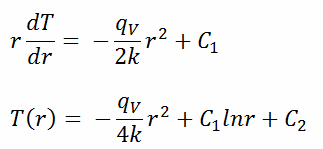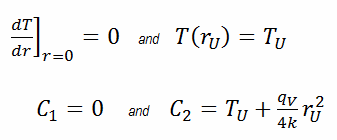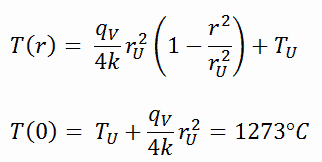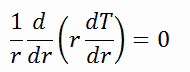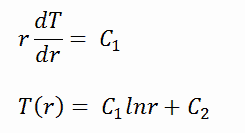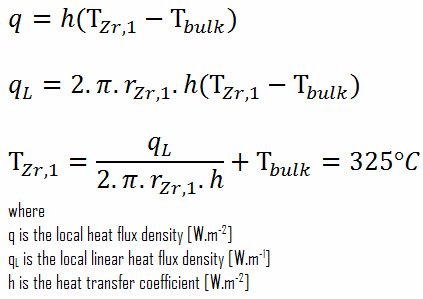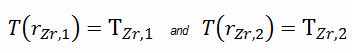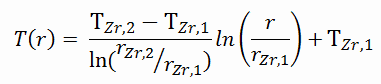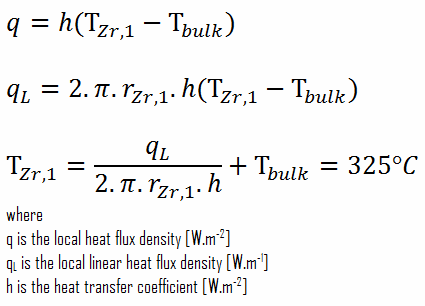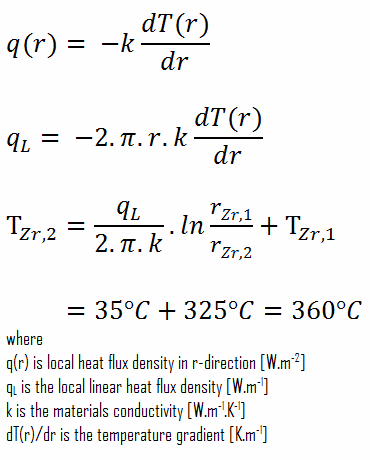Heat Conduction in a Large Plane Wall
Example of Heat Equation – Problem with Solution
Consider the plane wall of thickness 2L, in which there is uniform and constant heat generation per unit volume, qV [W/m3]. The center plane is taken as the origin for x, and the slab extends to + L on the right and – L on the left. For constant thermal conductivity k, the appropriate form of the heat equation is:
The general solution of this equation is:
where C1 and C2 are the constants of integration.
1)
 Calculate the temperature distribution, T(x), through this thick plane wall, if:
Calculate the temperature distribution, T(x), through this thick plane wall, if:
- the temperatures at both surfaces are 15.0°C
- the thickness of this wall is 2L = 10 mm.
- the material’s conductivity is k = 2.8 W/m.K (corresponds to uranium dioxide at 1000°C)
- the volumetric heat rate is qV = 106 W/m3
In this case, the surfaces are maintained at given temperatures Ts,1 and Ts,2. This corresponds to the Dirichlet boundary condition. Moreover, this problem is thermally symmetric, and therefore we may also use thermal symmetry boundary condition. The constants may be evaluated using substitution into the general solution and are of the form:
The resulting temperature distribution and the centerline (x = 0) temperature (maximum) in this plane wall at these specific boundary conditions will be:
The heat flux at any point, qx [W.m-2], in the wall may, of course, be determined by using the temperature distribution and with Fourier’s law. Note that, with heat generation, the heat flux is no longer independent of x, the, therefore:
Example of Heat Equation – Problem with Solution
Heat Conduction in a Fuel Rod
Most PWRs use uranium fuel, which is in the form of uranium dioxide. Uranium dioxide is a black semiconducting solid with very low thermal conductivity. On the other hand, uranium dioxide has a very high melting point and has well-known behavior. The UO2 is pressed into cylindrical pellets, and these pellets are then sintered into the solid.
These cylindrical pellets are then loaded and encapsulated within a fuel rod (or fuel pin) made of zirconium alloys due to their very low absorption cross-section (unlike stainless steel). The surface of the tube, which covers the pellets, is called fuel cladding.
See also: Thermal Conduction of Uranium Dioxide
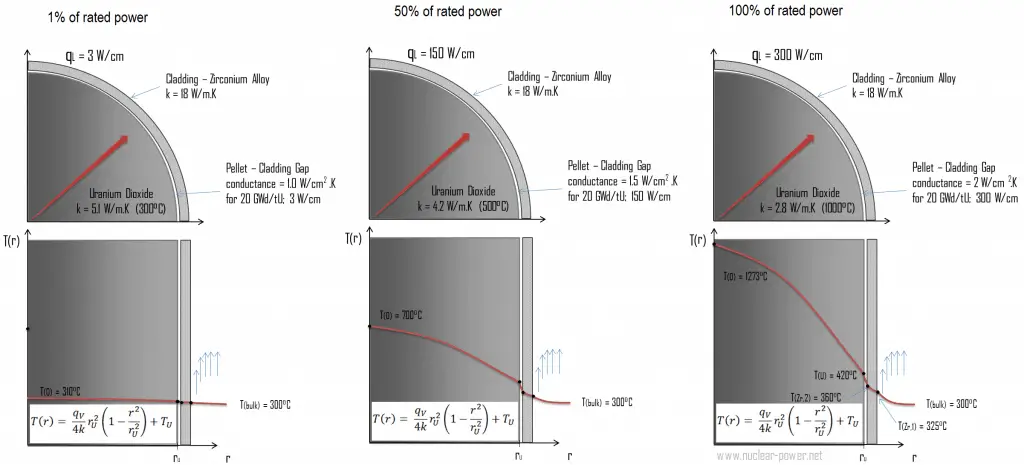
The thermal and mechanical behavior of fuel pellets and fuel rods constitute one of three key core design disciplines. Nuclear fuel is operated under inhospitable conditions (thermal, radiation, mechanical) and must withstand more than normal conditions operation. For example, temperatures in the center of fuel pellets reach more than 1000°C (1832°F), accompanied by fission-gas releases. Therefore detailed knowledge of temperature distribution within a single fuel rod is essential for the safe operation of nuclear fuel. This section will study the heat conduction equation in cylindrical coordinates using Dirichlet boundary condition with given surface temperature (i.e., using Dirichlet boundary condition). Comprehensive analysis of fuel rod temperature profile will be studied separately.
The temperature in the centerline of a fuel pellet
Consider the fuel pellet of radius rU = 0.40 cm, in which there is uniform and constant heat generation per unit volume, qV [W/m3]. Instead of volumetric heat rate qV [W/m3], engineers often use the linear heat rate, qL [W/m], representing the heat rate of one meter of a fuel rod. The linear heat rate can be calculated from the volumetric heat rate by:
The centreline is taken as the origin for r-coordinate. Due to symmetry in the z-direction and azimuthal direction, we can separate variables and simplify this problem to a one-dimensional problem. Thus, we will only solve for the temperature as a function of radius, T(r). For constant thermal conductivity, k, the appropriate form of the cylindrical heat equation, is:
The general solution of this equation is:
where C1 and C2 are the constants of integration.
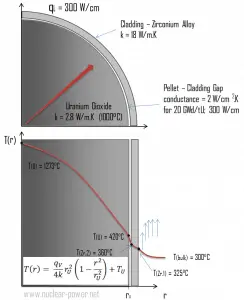 Calculate the temperature distribution, T(r), in this fuel pellet, if:
Calculate the temperature distribution, T(r), in this fuel pellet, if:
- the temperature at the surface of the fuel pellet is TU = 420°C
- the fuel pellet radius rU = 4 mm.
- the averaged material’s conductivity is k = 2.8 W/m.K (corresponds to uranium dioxide at 1000°C)
- the linear heat rate is qL = 300 W/cm and thus the volumetric heat rate is qV = 597 x 106 W/m3
In this case, the surface is maintained at given temperatures TU. This corresponds to the Dirichlet boundary condition. Moreover, this problem is thermally symmetric, and therefore we may also use thermal symmetry boundary condition. The constants may be evaluated using substitution into the general solution and are of the form:
The resulting temperature distribution and the centerline (r = 0) temperature (maximum) in this cylindrical fuel pellet at these specific boundary conditions will be:
The radial heat flux at any radius, qr [W.m-1], in the cylinder may, of course, be determined by using the temperature distribution and with Fourier’s law. Note that, with heat generation, the heat flux is no longer independent of r.
The following figure shows the temperature distribution in the fuel pellet at various power levels.
______
The temperature in an operating reactor varies from point to point within the system. Consequently, there is always one fuel rod and one local volume hotter than all the rest. Peak power limits must be introduced to limit these hot places. The peak power limits are associated with a boiling crisis and conditions that could cause fuel pellet melt. However, metallurgical considerations place upper limits on the fuel cladding temperature and the fuel pellet. Above these temperatures, there is a danger that the fuel may be damaged. One of the major objectives in the design of nuclear reactors is to remove the heat produced at the desired power level while assuring that the maximum fuel temperature and the maximum cladding temperature are always below these predetermined values.
Temperature distribution in Fuel Cladding
Cladding is the outer layer of the fuel rods, standing between the reactor coolant and the nuclear fuel (i.e., fuel pellets). It is made of corrosion-resistant material with a low absorption cross-section for thermal neutrons, usually zirconium alloy. Cladding prevents radioactive fission products from escaping the fuel matrix into the reactor coolant and contaminating it. Cladding constitutes one of the barriers in the ‘defense-in-depth ‘approach.
Consider the fuel cladding of inner radius rZr,2 = 0.408 cm and outer radius rZr,1 = 0.465 cm. Compared to fuel pellet, there is almost no heat generation in the fuel cladding (cladding is slightly heated by radiation). All heat generated in the fuel must be transferred via conduction through the cladding, and therefore the inner surface is hotter than the outer surface.
To find the temperature distribution through the cladding, we must solve the heat conduction equation. Due to symmetry in the z-direction and azimuthal direction, we can separate variables and simplify this problem to a one-dimensional problem. Thus, we will only solve for the temperature as a function of radius, T(r). In this example, we will assume that there is strictly no heat generation within the cladding. For constant thermal conductivity, k, the appropriate form of the cylindrical heat equation, is:
The general solution of this equation is:
where C1 and C2 are the constants of integration.
1)
 Calculate the temperature distribution, T(r), in this fuel cladding, if:
Calculate the temperature distribution, T(r), in this fuel cladding, if:
- the temperature at the inner surface of the cladding is TZr,2 = 360°C
- the temperature of reactor coolant at this axial coordinate is Tbulk = 300°C
- the heat transfer coefficient (convection; turbulent flow) is h = 41 kW/m2.K.
- the averaged material’s conductivity is k = 18 W/m.K
- the linear heat rate of the fuel is qL = 300 W/cm, and thus the volumetric heat rate is qV = 597 x 106 W/m3
From the basic relationship for heat transfer by convection, we can calculate the outer surface of the cladding as:
As can be seen, in this case, we have given surface temperatures TZr,1 and TZr,2. This corresponds to the Dirichlet boundary condition. The constants may be evaluated using substitution into the general solution and are of the form:
Solving for C1 and C2 and substituting into the general solution, we then obtain:
∆T – cladding surface – coolant
Detailed knowledge of geometry, the outer radius of cladding, linear heat rate, convective heat transfer coefficient, and the coolant temperature determines ∆T between the coolant (Tbulk) and the cladding surface (TZr,1). Therefore we can calculate the cladding surface temperature (TZr,1) simply using Newton’s Law:
∆T in fuel cladding
Detailed knowledge of geometry, outer and inner radius of cladding, linear heat rate, and the cladding surface temperature (TZr,1) determine ∆T between outer and inner surfaces of cladding. Therefore we can calculate the inner cladding surface temperature (TZr,2) simply using Fourier’s Law: