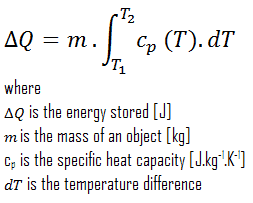The most direct way is the storage of sensible heat. Sensible heat storage is based on raising the temperature of a liquid or solid to store heat and releasing it with a decrease in temperature when required. The volumes needed to store energy on the scale that the world needs are extremely large. Materials used in sensible heat storage must have a high heat capacity and high boiling or melting point. Although this heat storage method is currently less efficient for heat storage, it is least complicated compared with latent or chemical heat, and it is inexpensive.
From a thermodynamics point of view, the storage of sensible heat is based on the increase of enthalpy of the material in the store, either a liquid or a solid, in most cases. The sensible effect is a temperature change. Heat stored can be obtained by the equation:
Heat Capacity
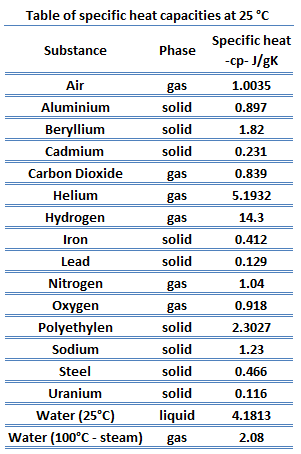 Different substances are affected to different magnitudes by the addition of heat. When a given amount of heat is added to different substances, their temperatures increase by different amounts. This proportionality constant between the heat Q that the object absorbs or loses and the resulting temperature change T of the object is known as the heat capacity C of an object.
Different substances are affected to different magnitudes by the addition of heat. When a given amount of heat is added to different substances, their temperatures increase by different amounts. This proportionality constant between the heat Q that the object absorbs or loses and the resulting temperature change T of the object is known as the heat capacity C of an object.
C = Q / ΔT
Heat capacity is an extensive property of matter, meaning it is proportional to the size of the system. Heat capacity C has the unit of energy per degree or energy per kelvin. When expressing the same phenomenon as an intensive property, the heat capacity is divided by the amount of substance, mass, or volume. Thus the quantity is independent of the size or extent of the sample.
Thermal Energy Storage
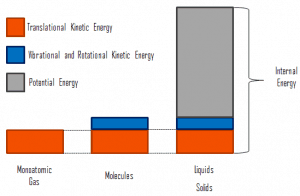 In thermodynamics, internal energy (also called thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, and it depends on the size of the system or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the system’s potential energy. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly. Still, techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
In thermodynamics, internal energy (also called thermal energy) is defined as the energy associated with microscopic forms of energy. It is an extensive quantity, and it depends on the size of the system or on the amount of substance it contains. The SI unit of internal energy is the joule (J). It is the energy contained within the system, excluding the kinetic energy of motion of the system as a whole and the system’s potential energy. Microscopic forms of energy include those due to the rotation, vibration, translation, and interactions among the molecules of a substance. None of these forms of energy can be measured or evaluated directly. Still, techniques have been developed to evaluate the change in the total sum of all these microscopic forms of energy.
In addition, energy can be stored in the chemical bonds between the atoms that make up the molecules. This energy storage on the atomic level includes energy associated with electron orbital states, nuclear spin, and binding forces in the nucleus.

Thermal energy can also be very effectively stored. Nowadays, the situation in energy markets is different. The increase in the prices of conventional energy sources and environmental awareness has led to an increase in the use of renewable energies and energy efficiency. Thermal energy storage forms a key component of a power plant to improve its dispatchability, especially for concentrating solar power plants (CSP). Thermal energy storage (TES) is achieved with widely differing technologies. There are three methods used and still being investigated to store thermal energy.
- Sensible Heat Storage (SHS)
- Latent Heat Storage (LHS)
- Thermo-chemical Storage