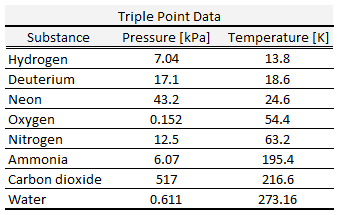
By international agreement, the triple point of water has been assigned a value of 273.16 K (0.01 °C; 32.02 °F) and partial vapor pressure of 611.66 pascals (6.1166 mbar; 0.0060366 atm).
At that point, it is possible to change all of the substances to vapor, water, or ice by making arbitrarily small changes in pressure and temperature. Even if the total pressure is well above the triple point of water, provided that the partial pressure of the water vapor is 611.657 pascals, then the system can still be brought to the triple point of water.
Source: wikipedia.org CC BY-SA
The triple point of water, T3 = 273.16 K, is the standard fixed-point temperature for the calibration of thermometers. This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of the water and absolute zero.
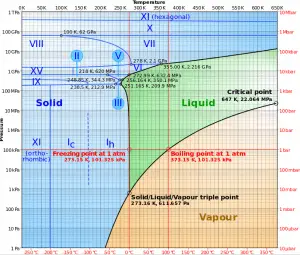
The phase diagram of water is a pressure-temperature diagram for water that shows how all three phases (solid, liquid, and vapor) may coexist together in thermal equilibrium. Along the vaporization line, the liquid and vapor phases are in equilibrium. The solid and liquid phases are in equilibrium along the fusion line, and along the sublimation line, the solid and vapor phases are in equilibrium. The only point at which all three phases may exist in equilibrium is the triple point.
The triple point of water corresponds to the minimum pressure at which water in the liquid state can exist. At pressures below the triple point (as in outer space), solid ice, when heated at constant pressure, is converted directly into water vapor in a process known as sublimation. In general, sublimation is a phase change of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase. When heated at constant pressure above the triple point, solid ice first melts to form liquid water and then evaporates to form water vapor.