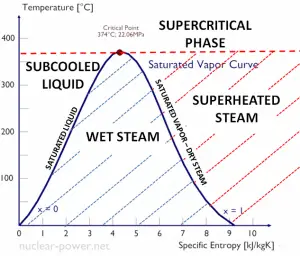
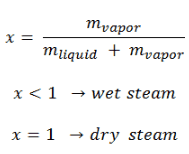If the temperature of the liquid is lower than the saturation temperature for the existing pressure, it is called either a subcooled liquid or a compressed liquid.
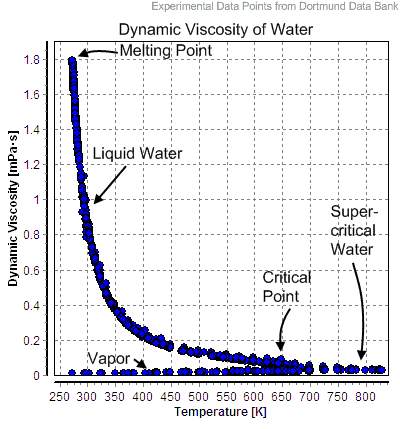
As seen from the phase diagram of water, in the two-phase regions (e.g.,, on the border of vapor/liquid phases), specifying temperature alone will set the pressure, and specifying pressure will set the temperature.
- The saturation vapor curve separates the two-phase state and the superheated vapor state in the T-s diagram.
- The saturated liquid curve separates the subcooled liquid state and the two-phase state in the T-s diagram.
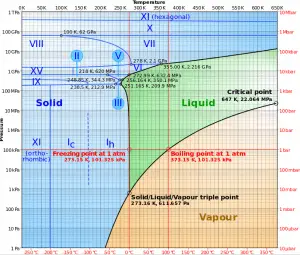
Source: wikipedia.org CC BY-SA
If water exists as a liquid at the saturation temperature and pressure with a quality of x = 0, it is called a saturated liquid state (single-phase). If the temperature of the liquid is lower than the saturation temperature for the existing pressure, it is called either a subcooled liquid or a compressed liquid. The term subcooling refers to a liquid existing at a temperature below its normal boiling point. For example, water normally boils at 100°C (at atmospheric pressure); at room temperature 20°C, the water is termed “subcooled”. Analogically the subcooling is also defined in nuclear engineering but for another purpose.
For example, the temperature in the pressurizer can be maintained at 350 °C (662 °F), which gives a subcooling margin (the difference between the pressurizer temperature and the highest temperature in the reactor core) of 30 °C. Subcooling margin is a very important safety parameter of PWRs since the boiling in the reactor core must be excluded.
Vapor Quality – Dryness Fraction
 As seen from the phase diagram of water, in the two-phase regions (e.g.,, on the border of vapor/liquid phases), specifying temperature alone will set the pressure, and specifying pressure will set the temperature. But these parameters will not define the volume and enthalpy because we will need to know the relative proportion of the two phases present.
As seen from the phase diagram of water, in the two-phase regions (e.g.,, on the border of vapor/liquid phases), specifying temperature alone will set the pressure, and specifying pressure will set the temperature. But these parameters will not define the volume and enthalpy because we will need to know the relative proportion of the two phases present.
The mass fraction of the vapor in a two-phase liquid-vapor region is called the vapor quality (or dryness fraction), x, and it is given by the following formula:
The value of the quality ranges from zero to unity. Although defined as a ratio, the quality is frequently given as a percentage. From this point of view, we distinguish between three basic types of steam. It must be added, at x=0, we are talking about the saturated liquid state (single-phase).
- Wet Steam
- Dry Steam
- Superheated Steam
This classification of steam has its limitation. Consider the system’s behavior, which is heated at a pressure that is higher than the critical pressure. In this case, there would be no change in phase from liquid to steam. In all states, there would be only one phase. Vaporization and condensation can occur only when the pressure is less than the critical pressure. The terms liquid and vapor tend to lose their significance.
See also: Saturation
Properties of Steam – Steam Tables
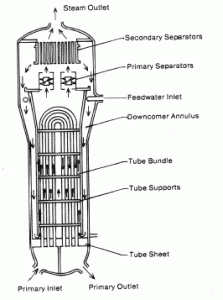
Water and steam are common fluids used for heat exchange in the primary circuit (from the surface of fuel rods to the coolant flow) and in the secondary circuit. It is used due to its availability and high heat capacity, both for cooling and heating. It is especially effective to transport heat through vaporization and condensation of water because of its very large latent heat of vaporization.
A disadvantage is that water-moderated reactors have to use the high-pressure primary circuit to keep water in the liquid state and achieve sufficient thermodynamic efficiency. Water and steam also react with metals commonly found in industries such as steel and copper, oxidized faster by untreated water and steam. In almost all thermal power stations (coal, gas, nuclear), water is used as the working fluid (used in a closed-loop between boiler, steam turbine, and condenser), and the coolant (used to exchange the waste heat to a water body or carry it away by evaporation in a cooling tower).
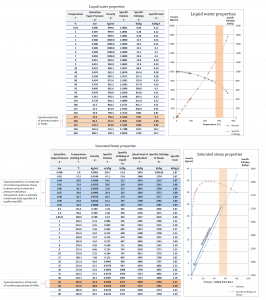
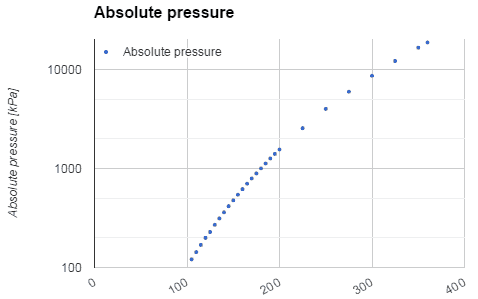
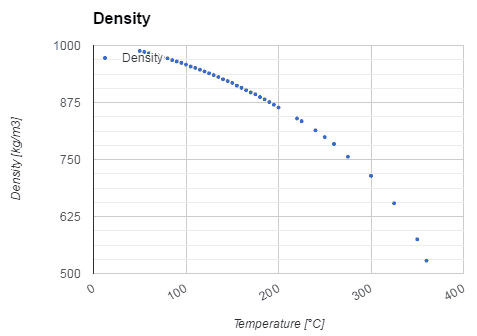
Water and steam are common medium because their properties are very well known. Their properties are tabulated in so-called “Steam Tables”. In these tables, the basic and key properties, such as pressure, temperature, enthalpy, density, and specific heat, are tabulated along the vapor-liquid saturation curve as a function of both temperature and pressure. The properties are also tabulated for single-phase states (compressed water or superheated steam) on a grid of temperatures and pressures extending to 2000 ºC and 1000 MPa.
Further comprehensive, authoritative data can be found at the NIST Webbook page on thermophysical properties of fluids.
See also: Steam Tables.
Special Reference: Allan H. Harvey. Thermodynamic Properties of Water, NISTIR 5078. Retrieved from https://www.nist.gov/sites/default/files/documents/srd/NISTIR5078.htm
Steam Tables
Special links:
www.nist.gov – Steam Tables – Saturation (Temperature)
www.nist.gov – Steam Tables – Saturation (Pressure)
www.nist.gov – Steam Tables – Compressed Water – Superheated Steam