Saturation
 In thermodynamics, the term saturation defines a condition in which a mixture of vapor and liquid can exist together at a given temperature and pressure. The temperature at which vaporization (boiling) occurs for a given pressure is called the saturation temperature or boiling point. The pressure at which vaporization (boiling) occurs for a given temperature is called the saturation pressure.
In thermodynamics, the term saturation defines a condition in which a mixture of vapor and liquid can exist together at a given temperature and pressure. The temperature at which vaporization (boiling) occurs for a given pressure is called the saturation temperature or boiling point. The pressure at which vaporization (boiling) occurs for a given temperature is called the saturation pressure.
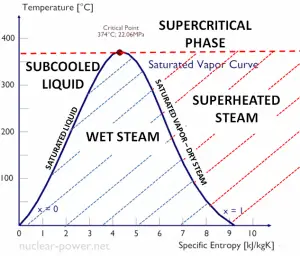
When the vapor quality is equal to 0, it is referred to as the saturated liquid state (single-phase). On the other hand, when the vapor quality is equal to 1, it is referred to as the saturated vapor state or dry steam (single-phase). Between these two states, we talk about vapor-liquid mixture or wet steam (two-phase mixture). At constant pressure, an addition of energy does not changes the temperature of the mixture, but the vapor quality and specific volume changes.
See also: Steam Tables.
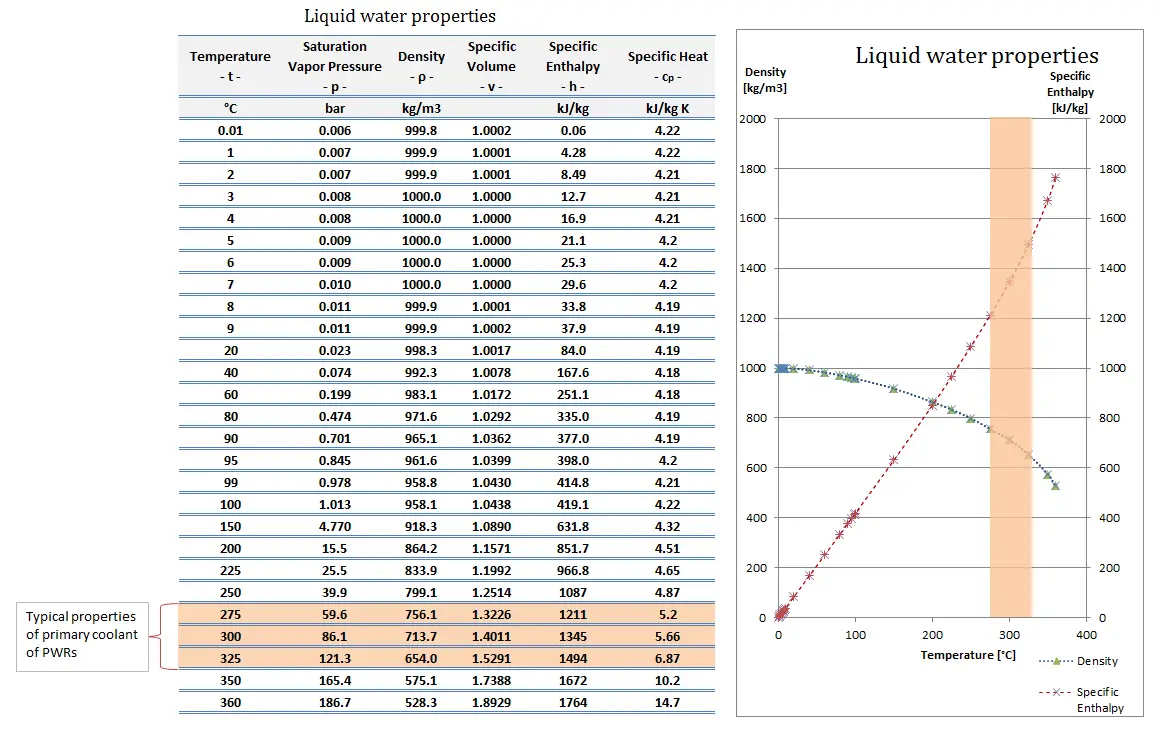
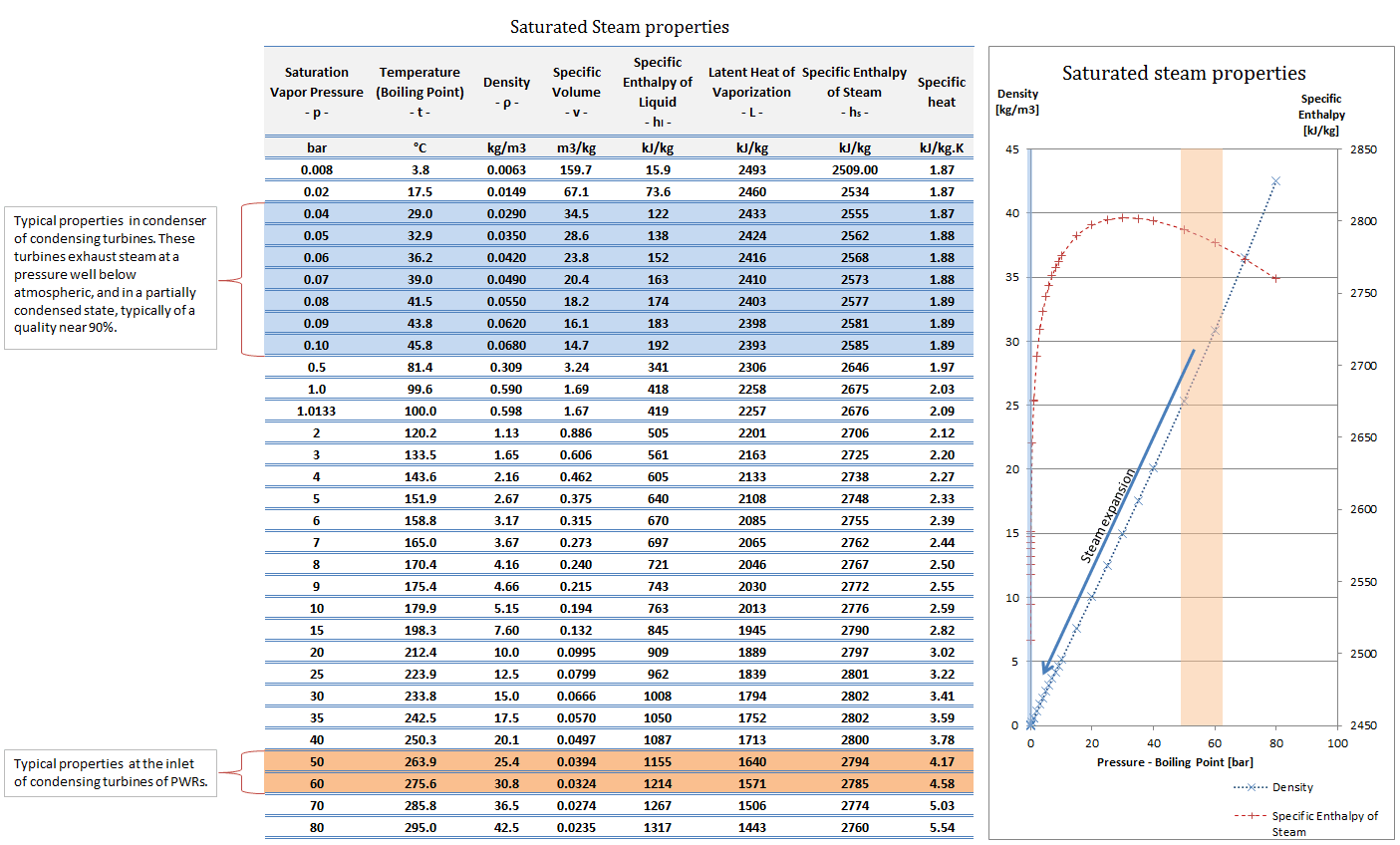
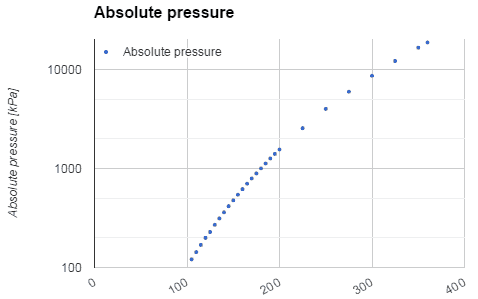
For example: In the pressurizer of pressurized water reactors, the saturation temperature is about 350°C, but this corresponds to the pressure of 16,4 MPa, which has to be maintained in the primary circuit. For a pure substance, there is a definite relationship between saturation pressure and saturation temperature. The higher the pressure, the higher the saturation temperature. The graphical representation of this relationship between temperature and pressure at saturated conditions is called the vapor pressure curve, and it can be seen in the phase diagram of water. This diagram is shown in the figure.
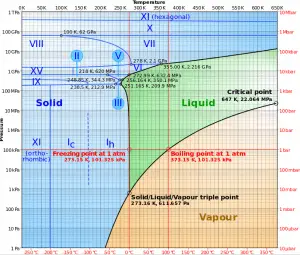
Source: wikipedia.org CC BY-SA
As seen from the phase diagram of water, in the two-phase regions (e.g.,, on the border of vapor/liquid phases), specifying temperature alone will set the pressure, and specifying pressure will set the temperature.
- The saturation vapor curve separates the two-phase state and the superheated vapor state in the T-s diagram.
- The saturated liquid curve separates the subcooled liquid state and the two-phase state in the T-s diagram.
Specific Enthalpy of Wet Steam
The specific enthalpy of saturated liquid water (x=0) and dry steam (x=1) can be picked from steam tables. In the case of wet steam, the actual enthalpy can be calculated with the vapor quality, x, and the specific enthalpies of saturated liquid water and dry steam:
hwet = hs x + (1 – x ) hl
where
hwet = enthalpy of wet steam (J/kg)
hs = enthalpy of “dry” steam (J/kg)
hl = enthalpy of saturated liquid water (J/kg)
As can be seen, wet steam will always have lower enthalpy than dry steam.