Source: wikipedia.org CC BY-SA
See also: Steam
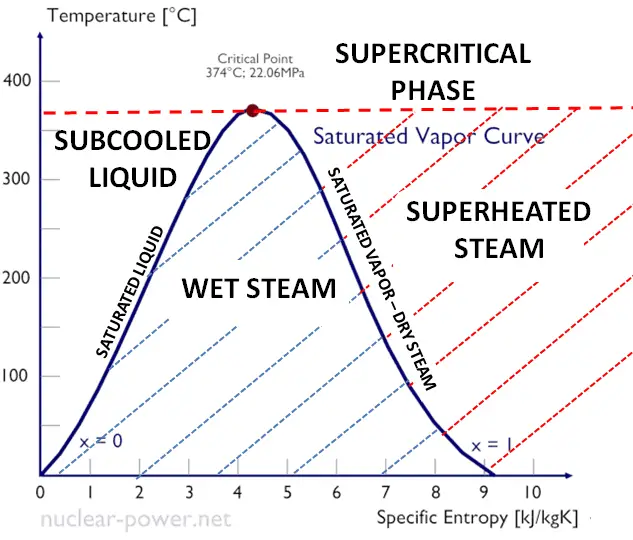
As seen from the phase diagram of water, in the two-phase regions (e.g.,, on the border of vapor/liquid phases), specifying temperature alone will set the pressure, and specifying pressure will set the temperature.
- The saturation vapor curve separates the two-phase state and the superheated vapor state in the T-s diagram.
- The saturated liquid curve separates the subcooled liquid state and the two-phase state in the T-s diagram.
Typically most nuclear power plants operate multi-stage condensing steam turbines. In these turbines, the high-pressure stage receives steam (this steam is nearly saturated steam – x = 0.995 – point C at the figure) from a steam generator and exhausts it to a moisture separator-reheater (point D). The steam must be reheated or superheated to avoid damages caused to the blades of the steam turbine by low-quality steam. High water droplets can cause rapid impingement and erosion of the blades when condensed water is blasted onto the blades. To prevent this, condensate drains are installed in the steam piping leading to the turbine. The reheater heats the steam (point D), and then the steam is directed to the low-pressure stage of the steam turbine, where it expands (point E to F). The exhausted steam is well below atmospheric pressure and is in a partially condensed state (point F), typically of a quality near 90%.