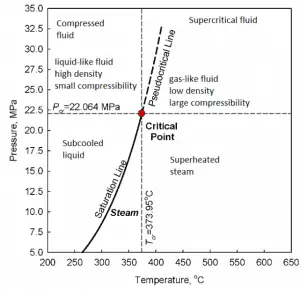
 A supercritical fluid is a fluid that is at pressures higher than its thermodynamic critical values. At the critical and supercritical pressures, a fluid is considered a single-phase substance, although all thermophysical properties undergo significant changes within the critical and pseudocritical regions.
A supercritical fluid is a fluid that is at pressures higher than its thermodynamic critical values. At the critical and supercritical pressures, a fluid is considered a single-phase substance, although all thermophysical properties undergo significant changes within the critical and pseudocritical regions.
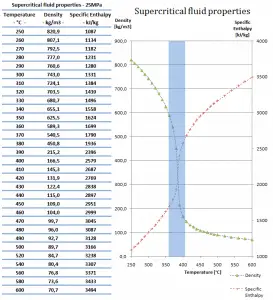
At pressures above the critical pressure, properties of water in the reactor change gradually and continuously from those we ordinarily associate with a liquid (high density, small compressibility) to those of a gas (low density, large compressibility) without a phase change. There is no change in the phase of water in the core. On the other hand, physical properties such as density, specific heat, specific enthalpy undergo significant changes, especially in the temperature range of the pseudocritical region (for 25 MPa between 372°C and 392°C). For example, in a typical supercritical water reactor:
- the density of supercritical water at the inlet and at the outlet is about 777 kg/m3 (for 25MPa and 280°C) and 90 kg/m3 (for 25MPa and 500°C),
- the specific enthalpy of supercritical water at the inlet and at the outlet is about 1230 kJ/kg (for 25MPa and 280°C) and 3165 kJ/kg (for 25MPa and 500°C)
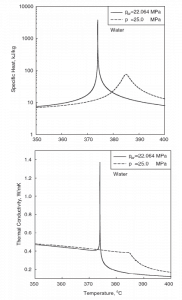 The following figures show the behavior of thermophysical properties of water near the critical (22.1MPa) and pseudocritical (25MPa) points. Near the critical point, these property changes are dramatic. In the vicinity of the pseudocritical point at 25 MPa, these property changes become less significant. At 25 MPa, the most significant property changes occur within ±25◦C around the pseudocritical point (389.4◦C). This region is known as the pseudocritical region. For convenience, fluid properties are considered to show liquid-like behavior below the pseudocritical point, and above the pseudocritical point, they are considered to show gas-like behavior.
The following figures show the behavior of thermophysical properties of water near the critical (22.1MPa) and pseudocritical (25MPa) points. Near the critical point, these property changes are dramatic. In the vicinity of the pseudocritical point at 25 MPa, these property changes become less significant. At 25 MPa, the most significant property changes occur within ±25◦C around the pseudocritical point (389.4◦C). This region is known as the pseudocritical region. For convenience, fluid properties are considered to show liquid-like behavior below the pseudocritical point, and above the pseudocritical point, they are considered to show gas-like behavior.