A chemical bond is a lasting attraction between these atoms, ions, or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. Therefore, the electromagnetic force plays a major role in determining the internal properties of most objects encountered in daily life.
Covalent Bond
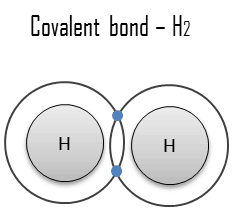 A covalent bond is a chemical bond formed by shared electrons. Valence electrons are shared when an atom needs electrons to complete its outer shell and can share those electrons with its neighbor. The electrons are then part of both atoms, filling both shells. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
A covalent bond is a chemical bond formed by shared electrons. Valence electrons are shared when an atom needs electrons to complete its outer shell and can share those electrons with its neighbor. The electrons are then part of both atoms, filling both shells. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
The simplest and most common type is a single bond in which two atoms share two electrons. Other types include the double bond (e.g., H2C=CH2), the triple bond, one- and three-electron bonds, the three-center two-electron bond, and the three-center four-electron bond.
Covalency is greatest between atoms of similar electronegativities. Thus, covalent bonding does not necessarily require that the two atoms be of the same elements, only that they be of comparable electronegativity (i.e., elements that lie near one another in the periodic table). There is no precise value that distinguishes ionic from covalent bonding. Still, an electronegativity difference of over 1.7 is likely to be ionic, while a difference of less than 1.7 is likely to be covalent.