A chemical bond is a lasting attraction between these atoms, ions, or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. Therefore, the electromagnetic force plays a major role in determining the internal properties of most objects encountered in daily life.
Metallic Bond
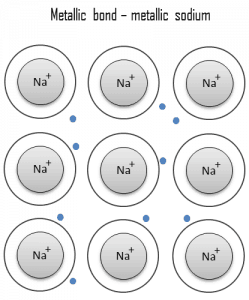 A metallic bond is a chemical bond in which the atoms do not share or exchange electrons to bond together. Instead, many electrons (roughly one for each atom) are more or less free to move throughout the metal so that each electron can interact with many fixed atoms. The free electrons shield the positively charged ion cores from the mutually repulsive electrostatic forces they would otherwise exert upon one another; consequently, the metallic bond is nondirectional in character. Metallic bonding is found in metals and their alloys. The free movement or delocalization of bonding electrons leads to classical metallic properties such as luster (surface light reflectivity), electrical and thermal conductivity, ductility, and high tensile strength.
A metallic bond is a chemical bond in which the atoms do not share or exchange electrons to bond together. Instead, many electrons (roughly one for each atom) are more or less free to move throughout the metal so that each electron can interact with many fixed atoms. The free electrons shield the positively charged ion cores from the mutually repulsive electrostatic forces they would otherwise exert upon one another; consequently, the metallic bond is nondirectional in character. Metallic bonding is found in metals and their alloys. The free movement or delocalization of bonding electrons leads to classical metallic properties such as luster (surface light reflectivity), electrical and thermal conductivity, ductility, and high tensile strength.
Metal is a material (usually solid) comprising one or more metallic elements (e.g., iron, aluminum, copper, chromium, titanium, gold, nickel), and often also nonmetallic elements (e.g., carbon, nitrogen, oxygen) in relatively small amounts. The unique feature of metals, as far as their structure is concerned, is the presence of charge carriers, specifically electrons. This feature is given by the nature of the metallic bond. The electrical and thermal conductivities of metals originate from their outer electrons being delocalized.