Understanding material science is essential for power plant personnel to understand why the material was selected for certain applications within their facility. Almost all processes that take place in nuclear facilities involve the use of specialized metals. To safely operate and maintain the facility and facility support systems, a basic understanding of material science is necessary for nuclear facility operators, maintenance personnel, and the technical staff. Our goal here will be to introduce the material engineering of nuclear reactors. The knowledge of thermophysical and nuclear properties of materials is essential for designing nuclear power plants.
Classification of Materials
A material is defined as a substance (most often a solid, but other condensed phases can be included) that is intended to be used for certain applications. There is a myriad of materials around us – they can be found in anything from buildings to spacecraft. Based on chemistry and atomic structure, materials are classified into three general categories:
-
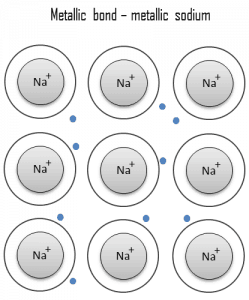
Metallic bond Metals. Metal is a material (usually solid) comprising one or more metallic elements (e.g., iron, aluminum, copper, chromium, titanium, gold, nickel), and often also nonmetallic elements (e.g., carbon, nitrogen, oxygen) in relatively small amounts. The unique feature of metals, as far as their structure is concerned, is the presence of charge carriers, specifically electrons. This feature is given by the nature of the metallic bond. In a metallic bond, the atoms do not share or exchange electrons to bond together. Instead, many electrons (roughly one for each atom) are more or less free to move throughout the metal so that each electron can interact with many fixed atoms. The electrical and thermal conductivities of metals originate from their outer electrons being delocalized.
- Ceramics. A ceramic is a solid material comprising an inorganic compound of metal, non-metal, or metalloid atoms primarily held in ionic and covalent bonds. Common examples are earthenware, porcelain, and brick. In the nuclear industry, uranium dioxide is a ceramic refractory uranium compound, in many cases, used as a nuclear fuel.
- Polymers. Polymers are compounds (macromolecules) composed of carbon, hydrogen, and other nonmetallic elements. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Some common and familiar polymers are polyethylene (PE), nylon, polycarbonate (PC), polystyrene (PS), and silicone rubber.
In addition, composites are composed of at least two different material types. Another materials category is the advanced materials that are used in high-tech applications, including:
- Semiconductors. In general, semiconductors are inorganic or organic materials that can control their conduction depending on chemical structure, temperature, illumination, and the presence of dopants. The name semiconductor comes from the fact that these materials have electrical conductivity between a metal, like copper, gold, etc., and an insulator, like glass.
- Biomaterials. In general, a biomaterial is any substance engineered to interact with biological systems for a medical purpose. These materials must not produce toxic substances and must be compatible with body tissues (i.e., must not cause adverse biological reactions).
- Smart materials. Smart materials sense and respond to changes in their environments in predetermined manners.
- Nanomaterials are materials with structural features on the order of a nanometer, some of which may be designed on the atomic/molecular level.