As was written, the crystalline material is one in which the atoms are situated in a repeating or periodic array over large atomic distances. That is, long-range order exists. Upon solidification, the atoms will position themselves in a repetitive three-dimensional pattern, in which each atom is bonded to its nearest neighbor atoms. But the reality is different, and real crystals are never perfect. There are always defects. The influence of these defects is not always adverse, and specific characteristics are often deliberately fashioned by introducing controlled amounts or numbers of particular defects.
Frenkel Defects
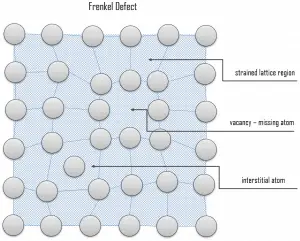 A Frenkel defect or a Frenkel pair is a nearby pair of a vacancy and an interstitial defect. This is caused when an ion moves into an interstitial site and creates a vacancy. Their primary mechanism of generation is particle irradiation. Frenkel defects are typical for radiation damage caused by high-energy neutrons. A 1 MeV neutron may affect approximately 5000 atoms. The presence of many displacement spikes will change the properties of the material being irradiated. A displacement spike contains large numbers of interstitials and lattice vacancies.
A Frenkel defect or a Frenkel pair is a nearby pair of a vacancy and an interstitial defect. This is caused when an ion moves into an interstitial site and creates a vacancy. Their primary mechanism of generation is particle irradiation. Frenkel defects are typical for radiation damage caused by high-energy neutrons. A 1 MeV neutron may affect approximately 5000 atoms. The presence of many displacement spikes will change the properties of the material being irradiated. A displacement spike contains large numbers of interstitials and lattice vacancies.