As was written, the crystalline material is one in which the atoms are situated in a repeating or periodic array over large atomic distances. That is, long-range order exists. Upon solidification, the atoms will position themselves in a repetitive three-dimensional pattern, in which each atom is bonded to its nearest neighbor atoms. But the reality is different, and real crystals are never perfect. There are always defects. The influence of these defects is not always adverse, and specific characteristics are often deliberately fashioned by introducing controlled amounts or numbers of particular defects.
Interstitial defects
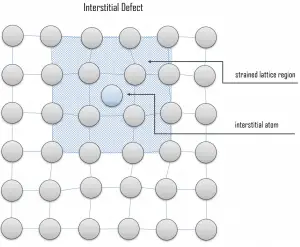 Interstitial defects result from an impurity located at an interstitial site or one of the lattice atoms being in an interstitial position instead of at its lattice position. A self-interstitial is an atom from the crystal crowded into an interstitial site. In metals, a self-interstitial introduces relatively large distortions and stress in the surrounding lattice because the atom is substantially larger than the interstitial position in which it is situated. Interstitial defects are generally high-energy configurations; on the other hand, the formation of this defect is not highly probable. Some crystals’ small atoms (mostly impurities) can occupy interstices without high energy, such as hydrogen.
Interstitial defects result from an impurity located at an interstitial site or one of the lattice atoms being in an interstitial position instead of at its lattice position. A self-interstitial is an atom from the crystal crowded into an interstitial site. In metals, a self-interstitial introduces relatively large distortions and stress in the surrounding lattice because the atom is substantially larger than the interstitial position in which it is situated. Interstitial defects are generally high-energy configurations; on the other hand, the formation of this defect is not highly probable. Some crystals’ small atoms (mostly impurities) can occupy interstices without high energy, such as hydrogen.