A solid solution is a uniform mixture of two crystalline solids that share a common crystal lattice. Solid solutions often consist of two or more types of atoms or molecules that share a crystal lattice, as in certain metal alloys. A solvent is an element or compound present in the greatest amount, and a solute is used to denote an element or compound in a minor concentration. The solute may incorporate into the solvent crystal lattice substitutionally by replacing a solvent particle in the lattice, or interstitially, by fitting into the space between solvent particles. Both types of solid solutions affect the material’s properties by distorting the crystal lattice and disrupting the physical and electrical homogeneity of the solvent material.
For the substitutional type, solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. According to these rules, substitutional solid solutions may form if the solute and solvent have:
- Similar atomic radii (15% or less difference)
- Same crystal structure
- Similar electronegativities
- Similar valency, a solid solution mixes with others to form a new solution
Solid solutions have important commercial and industrial applications, as such mixtures often have superior properties to pure materials. Many metal alloys are solid solutions. Even small amounts of solute can affect the electrical and physical properties of the solvent.
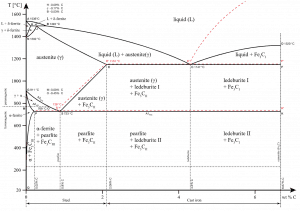
Alloying is a common practice because metallic bonds allow different types of metals to be joined. For example, austenitic stainless steels, including Type 304 stainless steel (containing 18%-20% chromium and 8%-10.5% nickel), have a face-centered cubic structure of iron atoms with the carbon in an interstitial solid solution.