As was written, the crystalline material is one in which the atoms are situated in a repeating or periodic array over large atomic distances. That is, long-range order exists. Upon solidification, the atoms will position themselves in a repetitive three-dimensional pattern, in which each atom is bonded to its nearest neighbor atoms. But the reality is different, and real crystals are never perfect. There are always defects. The influence of these defects is not always adverse, and specific characteristics are often deliberately fashioned by introducing controlled amounts or numbers of particular defects.
Substitutional defects
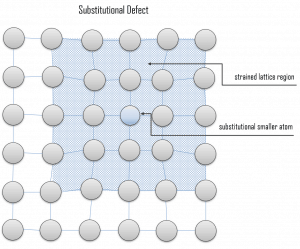 Due to fundamental limitations of material purification methods, materials are never 100% pure, which by definition induces defects in the crystal structure. Substitutional defects result from an impurity present at a lattice position. For the substitutional type, solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. According to these rules, substitutional solid solutions may form if the solute and solvent have:
Due to fundamental limitations of material purification methods, materials are never 100% pure, which by definition induces defects in the crystal structure. Substitutional defects result from an impurity present at a lattice position. For the substitutional type, solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. According to these rules, substitutional solid solutions may form if the solute and solvent have:
- Similar atomic radii (15% or less difference)
- Same crystal structure
- Similar electronegativities
- Similar valency, a solid solution mixes with others to form a new solution