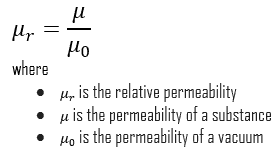Magnetic property refers to the response of a material to an applied magnetic field. The macroscopic magnetic properties of a material are a consequence of interactions between an external magnetic field and the magnetic dipole moments of the constituent atoms. Different materials react to the application of magnetic fields differently. The most familiar effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can be magnetized to become permanent magnets, producing magnetic fields. Only a few substances are ferromagnetic; the most common ones are iron, cobalt, and nickel and their alloys.
Types of Magnetism
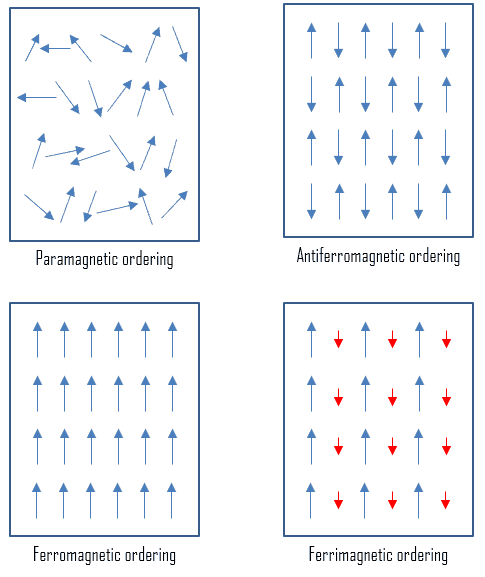 Five basic types of magnetism have been observed and classified based on the magnetic behavior of materials in response to magnetic fields at different temperatures. From this point of view, these types of materials are:
Five basic types of magnetism have been observed and classified based on the magnetic behavior of materials in response to magnetic fields at different temperatures. From this point of view, these types of materials are:
- Diamagnetic Material. Diamagnetic materials are those that some people generally think of as non-magnetic. A magnetic field repels diamagnetic materials; an applied magnetic field creates an induced magnetic field in the opposite direction, causing a repulsive force. Diamagnetism results from changes in electron orbital motion induced by an external field. Diamagnetic materials include water, wood, most organic compounds such as petroleum and some plastics, and many metals, including copper, particularly the heavy ones with many core electrons, such as mercury, gold, and bismuth. The effect is extremely small (with susceptibilities on the order of -10-5) and in opposition to the applied field. Diamagnetic materials, like water, or water-based materials, have a relative magnetic permeability that is less than or equal to 1 and, therefore, a magnetic susceptibility less than or equal to 0 since susceptibility is defined as χv = μv − 1. Diamagnetic and paramagnetic materials are considered non-magnetic because the magnetizations are relatively small and persist only while an applied field is present. If χ (magnetic susceptibility) is negative, the material is diamagnetic. In this case, the magnetic field in the material is weakened by the induced magnetization. Magnetic fields repel diamagnetic materials. For example, the magnetic susceptibility of diamagnets such as water is χv = −9.05×10−6. The most strongly diamagnetic material is bismuth, χv = −1.66×10−4. Generally, non-magnetic materials are said to be para- or diamagnetic because they do not possess permanent magnetization without an external magnetic field.
- Paramagnetic Materials. Paramagnetic materials have permanent atomic dipoles, which are acted on individually and aligned in the direction of an external field. Diamagnetic and paramagnetic materials are considered non-magnetic because the magnetizations are relatively small and persist only while an applied field is present. If χ (magnetic susceptibility) is positive, a material can be paramagnetic. In this case, the magnetic field in the material is strengthened by induced magnetization. Paramagnetic materials include most chemical elements and some compounds. They have a relative magnetic permeability slightly greater than 1 (i.e., a small positive magnetic susceptibility) and hence are attracted to magnetic fields. Generally, non-magnetic materials are said to be para- or diamagnetic because they do not possess permanent magnetization without an external magnetic field.
- Ferromagnetic Materials. Ferromagnetism is the basic mechanism by which material forms a permanent magnet (i.e., materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed). Ferromagnetism is the strongest type and is responsible for this common phenomenon. Ferromagnetic, ferrimagnetic, or antiferromagnetic materials possess permanent magnetization without an external magnetic field and do not have a well-defined zero-field susceptibility. Permanent magnetic moments in ferromagnetic materials result from atomic magnetic moments due to unpaired electron spins due to the electron structure. There is also a small orbital magnetic moment contribution compared to the spin moment. Thus, even in the absence of an applied field, the magnetic moments of the electrons in the material spontaneously line up parallel to one another. Every ferromagnetic material has its own individual temperature, called the Curie temperature, or Curie point, above which it loses its ferromagnetic properties. This is because the thermal tendency to disorder overwhelms the energy-lowering due to ferromagnetic order. Only a few substances are ferromagnetic. The common ones are iron, cobalt, nickel, most of their alloys, and some compounds of rare earth metals. Ferromagnetism is very important in industry and modern technology. Ferromagnetic materials can be divided into magnetically “soft” materials like annealed iron, which can be magnetized but does not tend to stay magnetized, and magnetically “hard” materials, which do. Permanent magnets are made from “hard” ferromagnetic materials such as alnico and ferrite that are subjected to special processing in a strong magnetic field during manufacture to align their internal microcrystalline structure, making them very hard to demagnetize.
- Antiferromagnetic Materials. In an antiferromagnet, unlike a ferromagnet, there is a tendency for the intrinsic magnetic moments of neighboring valence electrons to point in opposite directions. The substance is antiferromagnetic when all atoms are arranged in a substance so that each neighbor is anti-parallel. Antiferromagnets have a zero net magnetic moment, producing no field. Manganese oxide (MnO) is one material that displays this behavior. Generally, antiferromagnetic order may exist at sufficiently low temperatures but vanishes at and above the Néel temperature. Above the Néel temperature, the material is typically paramagnetic. That is, the thermal energy becomes large enough to destroy the microscopic magnetic ordering within the material. The Néel temperature of MnO is about 116K.
- Ferrimagnetic Materials. The macroscopic magnetic characteristics of ferromagnets and ferrimagnets are similar, and the distinction lies in the source of the net magnetic moments. A ferrimagnetic material has populations of atoms with opposing magnetic moments, as in antiferromagnetism; however, in ferrimagnetic materials, the opposing moments are unequal, and spontaneous magnetization remains. Ferromagnetic, ferrimagnetic, or antiferromagnetic materials possess permanent magnetization without an external magnetic field and do not have a well-defined zero-field susceptibility. Ferrites (widely used in household products such as refrigerator magnets) are usually ferrimagnetic ceramic compounds derived from iron oxides, and Magnetite (Fe3O4) is a famous example.
Generally, proper choice of materials is crucial in the main generator, where strong magnetic fields are present in nuclear power plants and power plants. In general, the main generator consists of a rotating part and a stationary part:
- Stator. The stator is the stationary part of an electric generator, which surrounds the rotor. The stator has a wire winding in which the changing field induces an electric current
- Rotor. The rotor is the rotating part of an electric generator and generates a magnetic field.
Permeability – Relative Permeability
In electromagnetism, permeability is the measure of the resistance of a substance against the formation of a magnetic field. The auxiliary magnetic field H represents how magnetic field B influences the organization of magnetic dipoles in a given substance. The magnetic field strength and flux density are related according to:
In this equation, B represents the magnitude of the internal field strength within a substance subjected to an H field. The permeability has dimensions of webers per ampere-meter (Wb/A.m) or henries per meter (H/m). The permeability constant μ0, also known as the magnetic constant or the permeability of free space, is a measure of the amount of resistance encountered when forming a magnetic field in a classical vacuum. This constant is very important since one of the important magnetic properties is the relative permeability (dimensionless), the ratio of the permeability in a material to the permeability in a vacuum.
According to the NIST reference on fundamental physical constants, the magnetic constant has the exact (defined) value
μ0 = 4π × 10−7 H/m ≈ 12.57×10−7 H/m.
A closely related property of materials is magnetic susceptibility, a dimensionless proportionality factor that indicates the degree of magnetization of a material in response to an applied magnetic field.
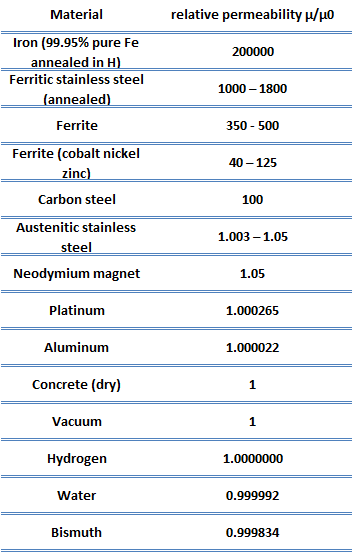 Neither μr nor χ is constant, as they can vary with the position in the medium. They depend not only on the material but also on the magnitude of the field, H, the frequency of the applied magnetic field, humidity, temperature, and other parameters. Nearly all materials respond to a magnetic field by becoming magnetized, but most are paramagnetic with a response so faint that it is of no practical use. A few, however, contain atoms that have large dipole moments and can spontaneously magnetize (i.e., to align their dipoles in parallel). These are called ferromagnetic and ferrimagnetic materials (the second one is called ferrites for short) and are of real practical use. Ferromagnetic, ferrimagnetic, or antiferromagnetic materials possess permanent magnetization without an external magnetic field and do not have a well-defined zero-field susceptibility.
Neither μr nor χ is constant, as they can vary with the position in the medium. They depend not only on the material but also on the magnitude of the field, H, the frequency of the applied magnetic field, humidity, temperature, and other parameters. Nearly all materials respond to a magnetic field by becoming magnetized, but most are paramagnetic with a response so faint that it is of no practical use. A few, however, contain atoms that have large dipole moments and can spontaneously magnetize (i.e., to align their dipoles in parallel). These are called ferromagnetic and ferrimagnetic materials (the second one is called ferrites for short) and are of real practical use. Ferromagnetic, ferrimagnetic, or antiferromagnetic materials possess permanent magnetization without an external magnetic field and do not have a well-defined zero-field susceptibility.
Magnetic Susceptibility
In electromagnetism, magnetic susceptibility is the measure of the magnetization of a substance. Magnetic susceptibility is a dimensionless proportionality factor that indicates the degree of magnetization of a material in response to an applied magnetic field. The magnitude of M is proportional to the applied field as follows:
The magnetic susceptibility and the relative permeability are related as follows:
 This allows a simple classification of most material’s response to an applied magnetic field into two categories: an alignment with the magnetic field, χ>0, called paramagnetism, or an alignment against the field, χ<0, called diamagnetism.
This allows a simple classification of most material’s response to an applied magnetic field into two categories: an alignment with the magnetic field, χ>0, called paramagnetism, or an alignment against the field, χ<0, called diamagnetism.
- Diamagnetic Material. Diamagnetic materials are those that some people generally think of as non-magnetic. A magnetic field repels diamagnetic materials; an applied magnetic field creates an induced magnetic field in the opposite direction, causing a repulsive force. Diamagnetism results from changes in electron orbital motion induced by an external field. Diamagnetic materials include water, wood, most organic compounds such as petroleum and some plastics, and many metals, including copper, particularly the heavy ones with many core electrons, such as mercury, gold, and bismuth. The effect is extremely small (with susceptibilities on the order of -10-5) and in opposition to the applied field. Diamagnetic materials, like water, or water-based materials, have a relative magnetic permeability that is less than or equal to 1 and, therefore, a magnetic susceptibility less than or equal to 0 since susceptibility is defined as χv = μv − 1. Diamagnetic and paramagnetic materials are considered non-magnetic because the magnetizations are relatively small and persist only while an applied field is present. If χ (magnetic susceptibility) is negative, the material is diamagnetic. In this case, the magnetic field in the material is weakened by the induced magnetization. Magnetic fields repel diamagnetic materials. For example, the magnetic susceptibility of diamagnets such as water is χv = −9.05×10−6. The most strongly diamagnetic material is bismuth, χv = −1.66×10−4. Generally, non-magnetic materials are said to be para- or diamagnetic because they do not possess permanent magnetization without an external magnetic field.
- Paramagnetic Materials. Paramagnetic materials have permanent atomic dipoles, which are acted on individually and aligned in the direction of an external field. Diamagnetic and paramagnetic materials are considered non-magnetic because the magnetizations are relatively small and persist only while an applied field is present. If χ (magnetic susceptibility) is positive, a material can be paramagnetic. In this case, the magnetic field in the material is strengthened by induced magnetization. Paramagnetic materials include most chemical elements and some compounds. They have a relative magnetic permeability slightly greater than 1 (i.e., a small positive magnetic susceptibility) and hence are attracted to magnetic fields. Generally, non-magnetic materials are said to be para- or diamagnetic because they do not possess permanent magnetization without an external magnetic field.
A closely related property of materials is the relative permeability, which is the ratio of the permeability in a material to the permeability in a vacuum. In general, permeability is the measure of the resistance of a substance against the formation of a magnetic field.
Neither μr nor χ is constants, as they can vary with the position in the medium. They depend not only on the material but also on the magnitude of the field, H, the frequency of the applied magnetic field, humidity, temperature, and other parameters. Nearly all materials respond to a magnetic field by becoming magnetized, but most are paramagnetic with a response so faint that it is of no practical use. A few, however, contain atoms that have large dipole moments and can spontaneously magnetize (i.e., to align their dipoles in parallel). These are called ferromagnetic and ferrimagnetic materials (the second one is called ferrites for short) and are of real practical use. Ferromagnetic, ferrimagnetic, or antiferromagnetic materials possess permanent magnetization without an external magnetic field and do not have a well-defined zero-field susceptibility.