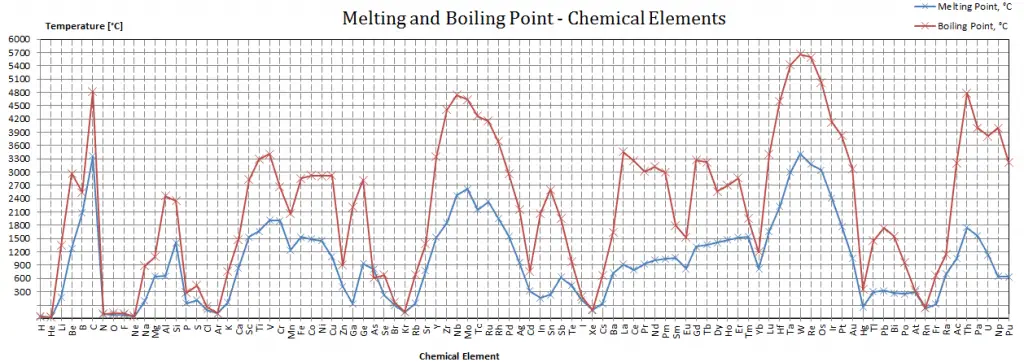
The temperature at which vaporization (boiling) starts to occur for a given pressure is also known as the saturation temperature. Under these conditions, a mixture of vapor and liquid can exist together. The liquid can be saturated with thermal energy and any addition of thermal energy results in a phase transition. At the boiling point, the two phases of a substance, liquid and vapor, have identical free energies and therefore are equally likely to exist. Below the boiling point, the liquid is the more stable state of the two, whereas the gaseous form is preferred above. The pressure at which vaporization (boiling) starts to occur for a given temperature is called the saturation pressure. When considered as the temperature of the reverse change from vapor to liquid, it is called the condensation point.
As can be seen, the boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. A liquid at high pressure has a higher boiling point than when that liquid is at atmospheric pressure. For example, water boils at 100°C (212°F) at sea level but 93.4°C (200.1°F) at 1900 meters (6,233 ft) altitude. On the other hand, water boils at 350°C (662°F) at 16.5 MPa (typical pressure of PWRs).
In the periodic table of elements, the element with the lowest boiling point is helium. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure. Since it is difficult to measure extreme temperatures without bias, both have been cited in the literature as having a higher boiling point.
Boiling point in the periodic table
For full interactivity, please visit material-properties.org.