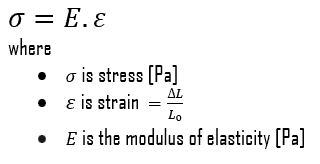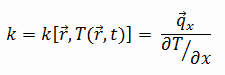Steel is an alloy of iron and carbon. Still, the term alloy steel usually only refers to steels that contain other elements— like vanadium, molybdenum, or cobalt—in amounts sufficient to alter the properties of the base steel. In general, alloy steel is alloyed with various elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties. Stainless steels are a specific group of high-alloy steels that contain a minimum of 11% chromium content by mass and a maximum of 1.2% carbon by mass. Alloy steels are broken down into two groups:
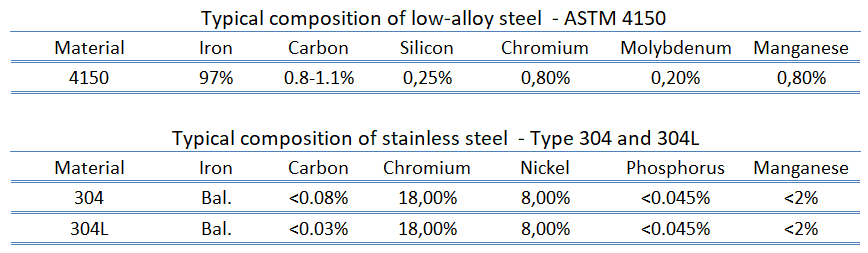 Low-alloy Steels. Low-alloy steels constitute a category of ferrous materials that exhibit mechanical properties superior to plain carbon steels resulting from additions of such alloying elements as nickel, chromium, molybdenum, manganese, and silicon. The role of the alloying elements is to increase hardenability to optimize mechanical properties and toughness after heat treatment. However, alloy additions sometimes reduce environmental degradation under specified service conditions.
Low-alloy Steels. Low-alloy steels constitute a category of ferrous materials that exhibit mechanical properties superior to plain carbon steels resulting from additions of such alloying elements as nickel, chromium, molybdenum, manganese, and silicon. The role of the alloying elements is to increase hardenability to optimize mechanical properties and toughness after heat treatment. However, alloy additions sometimes reduce environmental degradation under specified service conditions.- High-alloy Steels. Steels with alloying greater than 5 wt% are typically classified as high-alloy steel. Stainless steels are the major types of high-alloy steels, but two other types are ultrahigh-strength nickel-cobalt steels and maraging steels. Stainless steels are low-carbon high-alloy steels with at least 10.5% chromium with or without other alloying elements.
Special Reference: Metallurgy for the Non-metallurgist, 2nd Edition, ASM International. 450 pages, ISBN-10:1615038213.
Alloying Agents in Alloy Steels
Pure iron is too soft to be used for the purpose of structure, but adding small quantities of other elements (carbon, manganese, or silicon for instance) greatly increases its mechanical strength.

Alloys are usually stronger than pure metals, although they generally offer reduced electrical and thermal conductivity. Strength is the most important criterion by which many structural materials are judged. Therefore, alloys are used for engineering construction. The synergistic effect of alloying elements and heat treatment produces various microstructures and properties.
- Carbon. Carbon is a non-metallic element, an important alloying element in all ferrous metal-based materials. Carbon is always present in metallic alloys, i.e., in all grades of stainless steel and heat-resistant alloys. Carbon is a very strong austenitizing and increases the strength of steel. It is the principal hardening element essential to forming cementite, Fe3C, pearlite, spheroidite, and iron-carbon martensite. Adding a small amount of non-metallic carbon to iron trades its great ductility for greater strength. Suppose it combines chromium as a separate constituent (chromium carbide). In that case, it may have a detrimental effect on corrosion resistance by removing some of the chromium from the solid solution in the alloy and, consequently, reducing the amount of chromium available to ensure corrosion resistance.
- Chromium. Chromium increases hardness, strength, and corrosion resistance. The strengthening effect of forming stable metal carbides at the grain boundaries and the strong increase in corrosion resistance made chromium an important alloying material for steel. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 11% by weight, above which passivity can occur and below is impossible. Chromium can be used as a hardening element and is frequently used with a toughening element such as nickel to produce superior mechanical properties. At higher temperatures, chromium contributes to increased strength. The high-speed tool steels contain between 3 and 5% chromium, and it is ordinarily used for applications of this nature in conjunction with molybdenum.
- Nickel. Nickel is one of the most common alloying elements. About 65% of nickel production is used in stainless steel. Because nickel does not form any carbide compounds in steel, it remains in solution in the ferrite, thus strengthening and toughening the ferrite phase. Nickel steels are easily heat treated because nickel lowers the critical cooling rate. Nickel-based alloys (e.g., Fe-Cr-Ni(Mo) alloys) exhibit excellent ductility and toughness, even at high strength levels and these properties are retained up to low temperatures. Nickel also reduces thermal expansion for better dimensional stability. Nickel is the base element for superalloys, a group of nickel, iron-nickel, and cobalt alloys used in jet engines. These metals have excellent resistance to thermal creep deformation and retain their stiffness, strength, toughness, and dimensional stability at temperatures much higher than the other aerospace structural materials.
- Molybdenum. In small quantities in stainless steel, molybdenum increases hardenability and strength, particularly at high temperatures. The high melting point of molybdenum makes it important for giving strength to steel and other metallic alloys at high temperatures. Molybdenum is unique in the extent to which it increases steel’s high-temperature tensile and creeps strengths. It retards the transformation of austenite to pearlite far more than the transformation of austenite to bainite; thus, bainite may be produced by continuous cooling of molybdenum-containing steels.
- Vanadium. Vanadium is generally added to steel to inhibit grain growth during heat treatment. Controlling grain growth improves the strength and toughness of hardened and tempered steels.
- Tungsten. Tungsten produces stable carbides and refines grain size to increase hardness, particularly at high temperatures. Tungsten is used extensively in high-speed tool steels and has been proposed as a substitute for molybdenum in reduced-activation ferritic steels for nuclear applications.
Low-alloy Steels
Low-alloy steels constitute a category of ferrous materials that exhibit mechanical properties superior to plain carbon steels resulting from additions of such alloying elements as nickel, chromium, molybdenum, manganese, and silicon. The role of the alloying elements is to increase hardenability to optimize mechanical properties and toughness after heat treatment. However, alloy additions sometimes reduce environmental degradation under specified service conditions. Low-alloy steels may be classified into four major groups:
- low-carbon quenched and tempered (QT) steels
- medium-carbon ultrahigh-strength steels
- bearing steels
- heat-resistant chromium-molybdenum steels
41xx steel – Chromoly Steel – Medium-carbon Ultrahigh-strength Steels
Chromoly steel is a medium-carbon ultrahigh-strength low alloy steel that gets its name from the words “chromium” and “molybdenum” – two of the major alloying elements. Chromoly steel is often used when more strength is required than that mild carbon steel, though it often comes at an increase in cost. Chromoly falls under the AISI 41xx steel designations (ASTM A519). Examples of applications for 4130, 4140, and 4145 include structural tubing, bicycle frames, crankshafts, chain links, drill collars, gas bottles for transporting pressurized gases, firearm parts, clutch and flywheel components, and roll cages.
Properties of 41xx steel – Chromoly Steel
Material properties are intensive properties, which means they are independent of the amount of mass and may vary from place to place within the system at any moment. Materials science involves studying materials’ structure and relating them to their properties (mechanical, electrical, etc.). Once materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and how it has been processed into its final form.
Mechanical Properties of 41xx steel – Chromoly Steel
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial, and engineers must consider them.
Strength of 41xx steel – Chromoly Steel
In the mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. The strength of materials considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. The strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
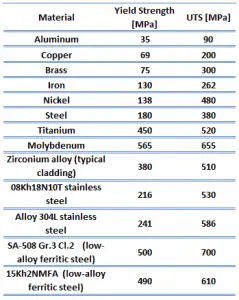
The ultimate tensile strength of 41xx steel – Chromoly steel depends on a certain grade, but it is about 700 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
Yield Strength
The yield strength of 41xx steel – Chromoly steel depends on a certain grade, but it is about 500 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically whereas yield point is the point where nonlinear (elastic + plastic) deformation begins. Before the yield point, the material will deform elastically and return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behavior termed a yield point phenomenon. Yield strengths vary from 35 MPa for low-strength aluminum to greater than 1400 MPa for high-strength steel.
Young’s Modulus of Elasticity
Young’s modulus of elasticity 41xx steel – Chromoly steel is 205 GPa.
Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to limiting stress, a body will be able to recover its dimensions on the removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position, and all the atoms are displaced the same amount and maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions, and no permanent deformation occurs. According to Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
The hardness of 41xx steel – Chromoly Steel
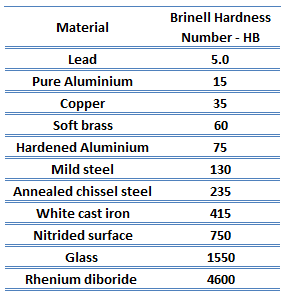
Brinell hardness of 41xx steel – Chromoly steel is approximately 200 MPa.
 In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Hardness is probably the most poorly defined material property because it may indicate resistance to scratching, abrasion, indentation, or even resistance to shaping or localized plastic deformation. Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness.
In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Hardness is probably the most poorly defined material property because it may indicate resistance to scratching, abrasion, indentation, or even resistance to shaping or localized plastic deformation. Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness.
Brinell hardness test is one of the indentation hardness tests developed for hardness testing. In Brinell tests, a hard, spherical indenter is forced under a specific load into the surface of the metal to be tested. The typical test uses a 10 mm (0.39 in) diameter hardened steel ball as an indenter with a 3,000 kgf (29.42 kN; 6,614 lbf) force. The load is maintained constant for a specified time (between 10 and 30 s). For softer materials, a smaller force is used; for harder materials, a tungsten carbide ball is substituted for the steel ball.
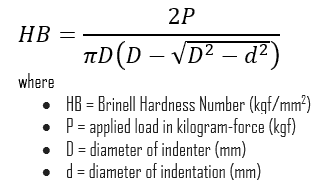
The test provides numerical results to quantify the hardness of a material, which is expressed by the Brinell hardness number – HB. The Brinell hardness number is designated by the most commonly used test standards (ASTM E10-14[2] and ISO 6506–1:2005) as HBW (H from hardness, B from Brinell, and W from the material of the indenter, tungsten (wolfram) carbide). In former standards, HB or HBS were used to refer to measurements made with steel indenters.
The Brinell hardness number (HB) is the load divided by the surface area of the indentation. The diameter of the impression is measured with a microscope with a superimposed scale. The Brinell hardness number is computed from the equation:
There are various test methods in common use (e.g., Brinell, Knoop, Vickers, and Rockwell). Some tables correlate the hardness numbers from the different test methods where correlation is applicable. In all scales, a high hardness number represents a hard metal.
Thermal Properties of 41xx steel – Chromoly Steel
Thermal properties of materials refer to the response of materials to changes in their temperature and to the application of heat. As a solid absorbs energy in the form of heat, its temperature rises, and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are often critical in solids’ practical use.
Melting Point of 41xx steel – Chromoly Steel
The melting point of 41xx steel – Chromoly steel is around 1427°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition where the solid and liquid can exist in equilibrium.
Thermal Conductivity of 41xx steel – Chromoly Steel
The thermal conductivity of 41xx steel – Chromoly steel is around 41 W/(m. K).
The heat transfer characteristics of solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies to all matter, regardless of its state (solid, liquid, or gas). Therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature, and for vapors, it also depends upon pressure. In general:
Most materials are nearly homogeneous. Therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material, the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.