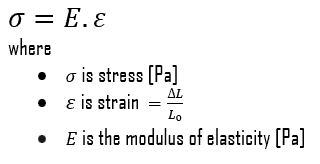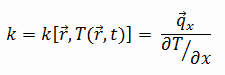High purity copper is a soft, malleable, and ductile metal with high thermal and electrical conductivity. A freshly exposed surface of pure copper has a reddish-orange color. Copper is used as a conductor of heat and electricity as a building material. As a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. High purity copper has the ultimate strength of approximately 210 MPa, and a yield strength of 33 Mpa, which limits its usability in industrial applications. But similarly, as for other alloys, copper may be strengthened. The main strengthening mechanism is alloying in Cu-based alloys.
 Copper alloys are alloys based on copper, in which the main alloying elements are Zn, Sn, Si, Al, and Ni. Cu-based alloys constitute mostly substitutional solid solutions, for which solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. There are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In addition, a limited number of copper alloys can be strengthened by heat treatment.; consequently, cold working and/or solid-solution alloying must be used to improve these mechanical properties.
Copper alloys are alloys based on copper, in which the main alloying elements are Zn, Sn, Si, Al, and Ni. Cu-based alloys constitute mostly substitutional solid solutions, for which solute or impurity atoms replace or substitute for the host atoms. Several features of the solute and solvent atoms determine the degree to which the former dissolves in the latter. These are expressed as the Hume–Rothery rules. There are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In addition, a limited number of copper alloys can be strengthened by heat treatment.; consequently, cold working and/or solid-solution alloying must be used to improve these mechanical properties.
Properties of Copper
Copper is a soft, tough, ductile, and malleable material, and these properties make copper extremely suitable for tube forming, wire drawing, spinning, and deep drawing. The other key properties exhibited by copper and its alloys include:
- Excellent thermal conductivity. Copper has a 60% higher thermal conductivity rating than aluminium, so it is better to reduce thermal hot spots in electrical wiring systems. The electrical and thermal conductivities of metals originate from their outer electrons being delocalized.
- Excellent electrical conductivity. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material for electricity transmission applications. However, aluminium is usually used in overhead high-voltage power lines because it has about half the weight and lowers the cost of a comparable resistance copper cable. At a given temperature, metals’ thermal and electrical conductivities are proportional, but raising the temperature increases the thermal conductivity while decreasing the electrical conductivity. This behavior is quantified in the Wiedemann–Franz law.
- Good corrosion resistance. Copper does not react with water but slowly reacts with atmospheric oxygen to form a layer of brown-black copper oxide. Unlike the rust that forms on iron in moist air, it protects the underlying metal from further corrosion (passivation). Copper-nickel alloys, aluminium brass, and aluminium demonstrate superior resistance to saltwater corrosion.
- Good biofouling resistance
- Good machinability. Machining of copper is possible, although alloys are preferred for good machinability in creating intricate parts.
- Retention of mechanical and electrical properties at cryogenic temperatures
- Diamagnetic
Uses of Copper and Copper Alloys
Historically, alloying copper with another metal, for example, tin, to make bronze was first practiced about 4000 years after the discovery of copper smelting and about 2000 years after “natural bronze” had come into general use. An ancient civilization is defined in the Bronze Age as producing bronze by smelting its copper and alloying it with tin, arsenic, or other metals. The major applications of copper are electrical wire (60%), roofing and plumbing (20%), and industrial machinery (15%). Copper is used mostly as a pure metal, but when greater hardness is required, it is put into such alloys as brass and bronze (5% of total use). Copper and copper-based alloys, including brasses (Cu-Zn) and bronzes (Cu-Sn), are widely used in different industrial and societal applications. Some brass alloys include costume jewelry, locks, hinges, gears, bearings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Bronze, or bronze-like alloys and mixtures, were used for coins over a longer period. It is still widely used today for springs, bearings, bushings, automobile transmission pilot bearings, and similar fittings and is particularly common in the bearings of small electric motors. Brass and bronze are common engineering materials in modern architecture and are primarily used for roofing and facade cladding due to their visual appearance.
Types of Copper Alloys
As was written, there are as many as 400 different copper and copper alloy compositions loosely grouped into the categories: copper, high copper alloy, brasses, bronzes, copper nickels, copper–nickel–zinc (nickel silver), leaded copper, and special alloys. In the following points, we summarize the key properties of selected copper-based materials.
 Electrolytic-tough pitch (ETP) copper. Electrolytic tough pitch copper, UNS C11000, is pure copper (with a maximum of 0.0355% impurities) refined by the electrolytic refining process. It is the most widely used grade of copper all over the world. ETP has a minimum conductivity rating of 100% IACS and is required to be 99.9% pure. It has 0.02% to 0.04% oxygen content (typical). Electrical wiring is the most important market for the copper industry. This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors. Pure copper has the best electrical and thermal conductivity of any commercial metal. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material for electricity transmission applications.
Electrolytic-tough pitch (ETP) copper. Electrolytic tough pitch copper, UNS C11000, is pure copper (with a maximum of 0.0355% impurities) refined by the electrolytic refining process. It is the most widely used grade of copper all over the world. ETP has a minimum conductivity rating of 100% IACS and is required to be 99.9% pure. It has 0.02% to 0.04% oxygen content (typical). Electrical wiring is the most important market for the copper industry. This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors. Pure copper has the best electrical and thermal conductivity of any commercial metal. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material for electricity transmission applications.- Brass. Brass is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion, and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow depending on the zinc content. Some common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and are primarily used for roofing and facade cladding due to their visual appearance.
- Bronze. The bronzes are a family of copper-based alloys traditionally alloyed with tin but can refer to alloys of copper and other elements (e.g., aluminum, silicon, and nickel). Bronzes are somewhat stronger than the brasses, yet they still have a high degree of corrosion resistance. Generally, they are used when good tensile properties are required in addition to corrosion resistance. For example, beryllium copper attains the greatest strength (to 1,400 MPa) of any copper-based alloy.
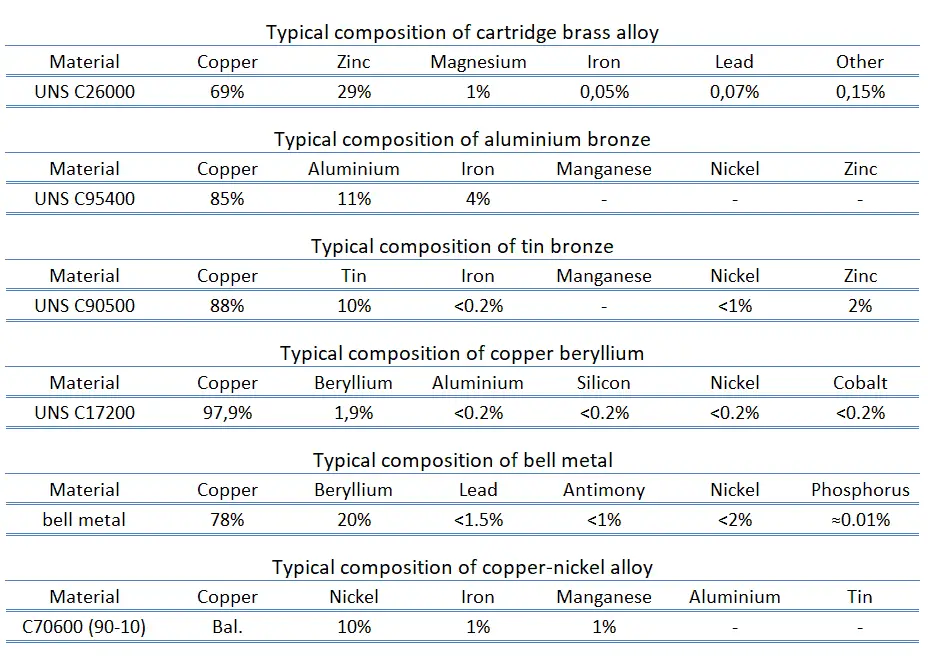
- Copper-nickel Alloy. Cupronickels are copper-nickel alloys that typically contain from 60 to 90 percent of copper and nickel as the main alloying element. The two main alloys are 90/10 and 70/30. Other strengthening elements, such as manganese and iron, maybe also contained. Cupronickels have excellent resistance to corrosion caused by seawater. Despite its high copper content, cupronickel is silver in color. Adding nickel to copper improves strength and corrosion resistance, but good ductility is retained.
- Nickel Silver. Nickel silver, also known as German silver, nickel brass, or alpaca, is a copper alloy with nickel and often zinc. For example, UNS C75700 nickel silver 65-12 copper alloy has good corrosion and tarnish resistance and high formability. Nickel silver is named due to its silvery appearance, but it contains no elemental silver unless plated.
Copper and Waste Management
Currently, the preferred option for the final disposal of high-level radioactive waste is a deep geological repository (DGR), an underground emplacement in stable geological formations. Crystalline rock (granite, welded tuff, and basalt), salt, and clay are the most suitable formations for geological disposal. The once-through cycle considers the spent nuclear fuel to be high-level waste (HLW) and, consequently, it is directly disposed of in a storage facility without being put through any chemical processes, where it will be safely stored for millions of years until its radiotoxicity reaches natural uranium levels or another safe reference level.
One possible option is to encapsulate this spent fuel in copper (CuOFP alloy – oxygen-free phosphorus-containing copper) capsules and deposit these canisters in a layer of bentonite clay, in a circular hole, eight meters deep and with a diameter of two meters, drilled in a cave 500 meters down into the crystalline rock. The deposits of native (pure) copper in the world have proven that the copper used in the final disposal container can remain unchanged inside the bedrock for extremely long periods if the geochemical conditions are appropriate (low levels of groundwater flow). The findings of ancient copper tools, many thousands of years old, also demonstrate the long-term corrosion resistance of copper, making it a credible container material for long-term radioactive waste storage.
Electrolytic-tough pitch (ETP) copper
Electrolytic tough pitch copper, UNS C11000, is pure copper (with a maximum of 0.0355% impurities) refined by the electrolytic refining process. It is the most widely used grade of copper all over the world. ETP has a minimum conductivity rating of 100% IACS and is required to be 99.9% pure. It has 0.02% to 0.04% oxygen content (typical). Electrical wiring is the most important market for the copper industry. This includes structural power wiring, power distribution cable, appliance wire, communications cable, automotive wire and cable, and magnet wire. Roughly half of all copper mined is used for electrical wire and cable conductors. Pure copper has the best electrical and thermal conductivity of any commercial metal. The conductivity of copper is 97% that of silver. Due to its much lower cost and greater abundance, copper has traditionally been the standard material for electricity transmission applications.
According to the Copper Development Association:
“The term ‘tough pitch’ originates from the time when molten copper, after refining, was cast into ingot moulds. During refining, the copper was oxidized to remove impurities and then reduced by hydrogen to give the correct oxygen level. To monitor this process, a small sample was taken, and the solidification surface was observed. If the surface sunk, there was too much oxygen; if it was raised, there was too much hydrogen. If it was level (correct pitch), the oxygen was correct, and the properties good; in other words,’ tough,’ hence tough pitch. “
Source: https://copperalliance.org.u
Brass
 Brass is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion, and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver, depending on the zinc content. Some common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and are primarily used for roofing and facade cladding due to their visual appearance.
Brass is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion, and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver, depending on the zinc content. Some common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins. Brass and bronze are common engineering materials in modern architecture and are primarily used for roofing and facade cladding due to their visual appearance.
For example, the UNS C26000 cartridge brass alloy (70/30) is from the yellow brass series, which has the highest ductility. Cartridge brasses are mostly cold formed and can also be easily machined, which is necessary in making cartridge cases. It can be used for radiator cores and tanks, flashlight shells, lamp fixtures, fasteners, locks, hinges, ammunition components, or plumbing accessories.
Bronze
The bronzes are a family of copper-based alloys traditionally alloyed with tin but can refer to alloys of copper and other elements (e.g., aluminum, silicon, and nickel). Bronzes are somewhat stronger than the brasses, yet they still have a high degree of corrosion resistance. Generally, they are used when good tensile properties are required in addition to corrosion resistance. For example, beryllium copper attains the greatest strength (to 1,400 MPa) of any copper-based alloy.
Historically, alloying copper with another metal, for example, tin, to make bronze was first practiced about 4000 years after the discovery of copper smelting and about 2000 years after “natural bronze” had come into general use. An ancient civilization is defined in the Bronze Age as producing bronze by smelting its copper and alloying it with tin, arsenic, or other metals. Bronze, or bronze-like alloys and mixtures, were used for coins over a longer period. It is still widely used today for springs, bearings, bushings, automobile transmission pilot bearings, and similar fittings and is particularly common in the bearings of small electric motors. Brass and bronze are common engineering materials in modern architecture and are primarily used for roofing and facade cladding due to their visual appearance.
Types of Bronzes
As was written, bronzes are a family of copper-based alloys traditionally alloyed with tin but can refer to alloys of copper and other elements (e.g., aluminum, silicon, and nickel).
- Tin and Phosphor Bronze. Bronzes are a family of copper-based alloys traditionally alloyed with tin, commonly with about 12–12.5% tin. The addition of small amounts (0.01–0.45) of phosphorus further increases the hardness, fatigue resistance, and wear resistance. The addition of these alloyants leads to applications such as springs, fasteners, masonry fixings, shafts, valve spindles, gears, and bearings. Bronze is also the preferred metal for bells in the form of a high tin bronze alloy known colloquially as bell metal, which is about 23% tin. High tin bronze alloys are typically found in gears and high-strength bushing and bearing applications where high strength and heavy loads are present. Other applications for these alloys are pump impellers, piston rings, and steam fittings. For example, copper casting alloy UNS C90500 is a cast alloy of copper-tin, also known as gunmetal. Originally used chiefly for making guns, it has largely been replaced by steel.
- Silicon Bronze. Silicon bronze usually contains about 96 percent copper. Silicon bronze has a composition of Si: 2.80–3.80%, Mn: 0.50–1.30%, Fe: 0.80% max., Zn: 1.50% max., Pb: 0.05% max. Silicon bronzes have a good combination of strength and ductility, good corrosion resistance, and easy weldability. Silicon bronzes were developed originally for the chemical industry because of their exceptional resistance to corrosion in many liquids. They are used in architectural product applications such as:
- Door fittings
- Railings
- Church doors
- Window frames
- Aluminium Bronze. The aluminum bronzes are a family of copper-based alloys that combine mechanical and chemical properties unmatched by any other alloy series. They contain about 5 to 12% of aluminium. In addition, aluminium bronzes also contain nickel, silicon, manganese, and iron. They have excellent strength, similar to low alloy steels, and excellent corrosion resistance, especially in seawater and similar environments, where the alloys often outperform many stainless steels. Their excellent resistance to corrosion results from the aluminium in the alloys, which reacts with atmospheric oxygen to form a thin, tough surface layer of alumina (aluminium oxide) which acts as a barrier to corrosion of the copper-rich alloy. They are found in wrought and cast form. Aluminium bronzes are usually golden in color. Aluminium bronzes are used in seawater applications that include:
- General sea water-related services
- Bearings
- Pipe fittings
- Pumps and valve components
- Heat exchangers
- Beryllium Bronze. Copper beryllium, also known as beryllium bronze, is a copper alloy with 0.5—3% beryllium. Copper beryllium is the hardest and strongest copper alloy (UTS up to 1,400 MPa) in fully heat treated, and cold worked conditions. It combines high strength with non-magnetic and non-sparking qualities. It is similar in mechanical properties to many high-strength alloy steels, but, compared to steels, it has better corrosion resistance. It has good thermal conductivity (210 W/m°C) 3-5 times more than tool steel. These high-performance alloys have long been used for non-sparking tools in the mining (coal mines), gas and petrochemical industries (oil rigs). Beryllium copper screwdrivers, pliers, wrenches, cold chisels, knives, and hammers are available for these environments. Because of the excellent fatigue resistance, copper beryllium is widely used for springs, spring wire, load cells, and other parts that must retain their shape under cyclic loads.
- Bell Metal (High-tin Bronze). In general, bell metals usually refer to high-tin bronzes that are a family of copper-based alloys traditionally alloyed with tin, commonly with more than 20% of tin (typically, 78% copper, 22% tin by mass). Bell metal is used for the casting of high-quality bells. The higher tin content increases the rigidity of the metal and the resonance. It has been found that increasing the tin content increases the decay time of the bell strike, thus making the bell more sonorous. High-tin bronzes are also found in gears and high-strength bushing and bearing applications where high strength and heavy loads are present.
Properties of Copper Alloys
Material properties are intensive properties, which means they are independent of the amount of mass and may vary from place to place within the system at any moment. Materials science involves studying materials’ structure and relating them to their properties (mechanical, electrical, etc.). Once materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and how it has been processed into its final form.
Mechanical Properties of Copper Alloys
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial, and engineers must consider them.
Strength of Copper Alloys
In the mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. The strength of materials considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. The strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
The ultimate tensile strength of electrolytic-tough pitch (ETP) copper is about 250 MPa.
The ultimate tensile strength of cartridge brass – UNS C26000 is about 315 MPa.
The ultimate tensile strength of aluminium bronze – UNS C95400 is about 550 MPa.
The ultimate tensile strength of tin bronze – UNS C90500 – gun metal is about 310 MPa.
The ultimate tensile strength of copper beryllium – UNS C17200 is about 1380 MPa.
The ultimate tensile strength of cupronickel – UNS C70600 is about 275 MPa.
The ultimate tensile strength of nickel silver – UNS C75700 is about 400 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the specimen preparation, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or “the ultimate.” If this stress is applied and maintained, a fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore, its value does not depend on the size of the test specimen. However, it depends on other factors, such as the specimen preparation, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steel.
Yield Strength
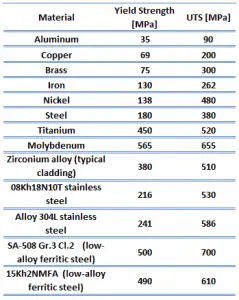
Proof strength of electrolytic-tough pitch (ETP) copper is between 60-300 MPa.
The yield strength of aluminium bronze – UNS C95400 is about 250 MPa.
The yield strength of tin bronze – UNS C90500 – gun metal is about 150 MPa.
The yield strength of copper beryllium – UNS C17200 is about 1100 MPa.
The yield strength of cupronickel – UNS C70600 is about 105 MPa.
The yield strength of nickel silver – UNS C75700 is about 170 MPa.
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically. In contrast, the yield point is the point where nonlinear (elastic + plastic) deformation begins. Before the yield point, the material will deform elastically and return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behavior termed a yield point phenomenon. Yield strengths vary from 35 MPa for low-strength aluminum to greater than 1400 MPa for high-strength steel.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of electrolytic-tough pitch (ETP) copper is about 120 GPa.
Young’s modulus of elasticity of cartridge brass – UNS C26000 is about 95 GPa.
Young’s modulus of elasticity of aluminium bronze – UNS C95400 is about 110 GPa.
Young’s modulus of elasticity of tin bronze – UNS C90500 – gun metal is about 103 GPa.
Young’s modulus of elasticity of copper beryllium – UNS C17200 is about 131 GPa.
Young’s modulus of elasticity of cupronickel – UNS C70600 is about 135 GPa.
Young’s modulus of elasticity of nickel silver – UNS C75700 is about 117 GPa.
Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to limiting stress, a body will be able to recover its dimensions on the removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position, and all the atoms are displaced the same amount and maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions, and no permanent deformation occurs. According to Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
The hardness of Copper Alloys
Vickers hardness of electrolytic-tough pitch (ETP) copper depends greatly on the temper of the material, but it is between 50 – 150 HV.
Brinell hardness of cartridge brass – UNS C26000 is approximately 100 MPa.
Brinell hardness of aluminium bronze – UNS C95400 is approximately 170 MPa. The hardness of aluminum bronzes increases with aluminum (and other alloys) content and stresses caused by cold working.
Brinell hardness of tin bronze – UNS C90500 – gun metal is approximately 75 BHN.
Rockwell hardness of copper beryllium – UNS C17200 is approximately 82 HRB.
Brinell hardness of cupronickel – UNS C70600 is approximately HB 100.
Rockwell hardness of nickel silver – UNS C75700 is approximately 45 HRB.
Rockwell hardness test is one of the most common indentation hardness tests developed for hardness testing. In contrast to the Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position, and the major load is applied and removed while maintaining the minor load. The difference between the penetration depth before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Copper Alloys
Thermal properties of materials refer to the response of materials to changes in their temperature and the application of heat. As a solid absorbs energy in the form of heat, its temperature rises, and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are often critical in solids’ practical use.
Melting Point of Copper Alloys
The melting point of electrolytic-tough pitch (ETP) copper is around 1085°C.
The melting point of cartridge brass – UNS C26000 is around 950°C.
The melting point of aluminium bronze – UNS C95400 is around 1030°C.
The melting point of tin bronze – UNS C90500 – gun metal is around 1000°C.
The melting point of copper beryllium – UNS C17200 is around 866°C.
The melting point of cupronickel – UNS C70600 is around 1100°C.
The melting point of nickel silver – UNS C75700 is around 1040°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition where the solid and liquid can exist in equilibrium.
Thermal Conductivity of Copper Alloys
The thermal conductivity of electrolytic-tough pitch (ETP) copper is 394 W/(m. K).
The thermal conductivity of cartridge brass – UNS C26000 is 120 W/(m. K).
The thermal conductivity of aluminium bronze – UNS C95400 is 59 W/(m. K).
The thermal conductivity of tin bronze – UNS C90500 – gun metal is 75 W/(m. K).
The thermal conductivity of copper beryllium – UNS C17200 is 115 W/(m. K).
The thermal conductivity of cupronickel – UNS C70600 is 40 W/(m. K).
The thermal conductivity of nickel silver – UNS C75700 is 40 W/(m. K).
The heat transfer characteristics of solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It measures a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies to all matter, regardless of its state (solid, liquid, or gas). Therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature, and for vapors, it also depends upon pressure. In general:
Most materials are nearly homogeneous. Therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz). However, for an isotropic material, the thermal conductivity is independent of the transfer direction, kx = ky = kz = k.
Electrical Conductivity of Copper Alloys
The electrical conductivity of electrolytic-tough pitch (ETP) copper is 101% IACS (around 58.6 MS/m).
The electrical conductivity of cartridge brass – UNS C26000 is about 30% IACS (around 17 MS/m).
Electrical resistivity and its converse, electrical conductivity, is a fundamental property of a material that quantifies how strongly it resists or conducts the flow of electric current. A low resistivity indicates a material that readily allows the flow of electric current. The symbol of resistivity is usually the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-meter (Ω⋅m). Note that electrical resistivity is not the same as electrical resistance. Electrical resistance is expressed in Ohms. While resistivity is a material property, resistance is the property of an object.