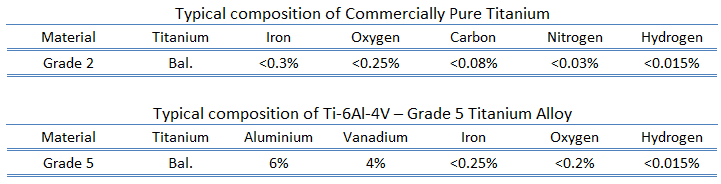
 Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in seawater, aqua regia, and chlorine. In power plants, titanium can be used in surface condensers. The Kroll and Hunter processes extract the metal from its principal mineral ores. Kroll’s process involved a reduction of titanium tetrachloride (TiCl4), first with sodium and calcium and later with magnesium, under an inert gas atmosphere. Pure titanium is stronger than common, low-carbon steels but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. Titanium’s corrosion resistance is based on forming a stable, protective oxide layer. Although “commercially pure” titanium has acceptable mechanical properties and has been used for orthopedic and dental implants, titanium is alloyed with small amounts of aluminium and vanadium, typically 6% and 4%, respectively, for most applications by weight. This mixture has a solid solubility that varies dramatically with temperature, allowing it to undergo precipitation strengthening.
Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in seawater, aqua regia, and chlorine. In power plants, titanium can be used in surface condensers. The Kroll and Hunter processes extract the metal from its principal mineral ores. Kroll’s process involved a reduction of titanium tetrachloride (TiCl4), first with sodium and calcium and later with magnesium, under an inert gas atmosphere. Pure titanium is stronger than common, low-carbon steels but 45% lighter. It is also twice as strong as weak aluminium alloys but only 60% heavier. The two most useful properties of the metal are corrosion resistance and strength-to-density ratio, the highest of any metallic element. The corrosion resistance of titanium alloys at normal temperatures is unusually high. Titanium’s corrosion resistance is based on forming a stable, protective oxide layer. Although “commercially pure” titanium has acceptable mechanical properties and has been used for orthopedic and dental implants, titanium is alloyed with small amounts of aluminium and vanadium, typically 6% and 4%, respectively, for most applications by weight. This mixture has a solid solubility that varies dramatically with temperature, allowing it to undergo precipitation strengthening.
 Grade 1 Titanium
Grade 1 Titanium
Commercially pure titanium grade 1 is the most ductile and softest titanium alloy. It is a good solution for cold-forming and corrosive environments. It possesses the greatest formability, excellent corrosion resistance, and high impact toughness. Due to its formability, it is commonly available as a titanium plate and tubing. These include:
- Chemical processing
- Chlorate manufacturing
- Architecture
- Medical industry
- Marine industry
- Automotive parts
- Airframe structure
Commercially Pure Titanium – Grade 1 in Steam Condensers
In nuclear power plants, the main steam condenser (MC) system is designed to condense and deaerate the exhaust steam from the main turbine and provide a heat sink for the turbine bypass system. The exhaust steam from the LP turbines is condensed by passing over tubes containing water from the cooling system. These tubes are usually made of stainless steel, copper alloys, or titanium, depending on several selection criteria (such as thermal conductivity or corrosion resistance). Titanium condenser tubes are usually the best technical choice. However, titanium is a very expensive material, and titanium condenser tubes are associated with very high initial costs. Titanium, in particular, can bring major improvements, such as higher water velocities promoting better heat coefficients and excellent resistance to abrasion, erosion, and corrosion, thereby improving resistance to fouling. Tubes are mostly welded tubes from ASTM SB 338 grade 1 made on a continuous manufacturing line. This commercially pure titanium is the softest titanium and has the highest ductility. It has a good cold forming characteristics and provides excellent corrosion resistance. It also has excellent welding properties and high impact toughness. All manufacturing operations (welding, annealing, non-destructive testing) are fully automated to produce high-quality tubes in large quantities.
Types of Titanium Alloys
Titanium exists in two crystallographic forms. At room temperature, unalloyed (commercially pure) titanium has a hexagonal close-packed (hcp) crystal structure referred to as the alpha (α) phase. When the temperature of pure titanium reaches 885 °C (called the β transit temperature of titanium), the crystal structure changes to a bcc structure known as the beta (β) phase. Alloying elements either raise or lower the temperature for the α-to- β transformation, so alloying elements in titanium are classified as either α stabilizers or β stabilizers. For example, vanadium, niobium, and molybdenum decrease the α-to-β transformation temperature and promote the formation of the β phase.
- Alpha Alloys. Alpha alloys contain elements such as aluminum and tin and are preferred for high-temperature applications because of their superior creep characteristics. These α-stabilizing elements work by either inhibiting change in the phase transformation temperature or by causing it to increase. The absence of a ductile-to-brittle transition, a feature of β alloys, makes α alloys suitable for cryogenic applications. On the other hand, it cannot be strengthened by heat treatment because alpha is the stable phase, and thus they are not as strength as beta alloys.
- Beta Alloys. Beta alloys contain transition elements such as vanadium, niobium, and molybdenum, which decrease the temperature of the α to β phase transition. Beta alloys have excellent hardenability and respond readily to heat treatment. These materials are highly forgeable and exhibit high fracture toughnesses. For example, the ultimate tensile strength of high-strength titanium alloy – TI-10V-2Fe-3Al is about 1200 MPa.
- Alpha + Beta Alloy. Alpha + beta alloys have compositions that support a mixture of α and β phases and may contain between 10 and 50% β phase at room temperature. The most common α + β alloy is Ti-6Al-4V. The strength of these alloys may be improved and controlled by heat treatment. Examples include: Ti-6Al-4V, Ti-6Al-4V-ELI, Ti-6Al-6V-2Sn, Ti-6Al-7Nb.