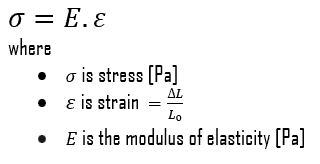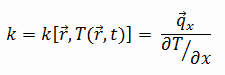Zinc is a brittle metal with a relatively low melting point of 419 °C (787 °F), resists corrosion, is ductile and malleable, and is highly soluble in copper. Zinc and zinc alloys are used in the form of coatings, castings, rolled sheets, drawn wire, forgings, and extrusions. Other uses of zinc are a major constituent in brass nickel-silver alloys, typewriter metal, soft and aluminium solder, and commercial bronze.
Alloys of zinc with small amounts of copper, aluminium, and magnesium are useful in die casting and spin casting, especially in the automotive, electrical, and hardware industries. Zinc alloys have low melting points, require relatively low heat input, and do not require fluxing or protective atmospheres. Because of their high fluidity, zinc alloys can be cast in much thinner walls than other die castings alloys, and they can be die cast to tighter dimensional tolerances. These zinc alloys are marketed under the name Zamak. The name Zamak is an acronym of the German names for the metals of which the alloys are composed: Zink (zinc), Aluminium, Magnesium, and Kupfer (copper). The low melting point and the low viscosity of the alloy make possible the production of small and intricate shapes.
As a coating, zinc provides corrosion protection for iron and steel (galvanized steel). Steel coating constitutes the largest single use of zinc, but it is used in large tonnages in zinc alloy castings, as zinc dust and oxide, and in wrought zinc products. Galvanized steel is just plain carbon steel coated with a thin zinc layer. The zinc protects iron by corroding first, but zinc corrodes at much lower rates than steel. In the event the underlying metal becomes exposed, protection can continue as long as there is zinc close enough to be electrically coupled. After all of the zinc in the immediate area is consumed, localized corrosion of the base metal can occur. Galvanized construction steels are the most common use for galvanized metal, and hundreds of thousands of tons of steel products are galvanized annually worldwide (sheet metal, fences, screens, screws, etc.).
Brass – Copper-Zinc Alloy
Brass is the generic term for a range of copper-zinc alloys. Brass can be alloyed with zinc in different proportions, which results in a material of varying mechanical, corrosion, and thermal properties. Increased amounts of zinc provide the material with improved strength and ductility. Brasses with a copper content greater than 63% are the most ductile of any copper alloy and are shaped by complex cold-forming operations. Brass has higher malleability than bronze or zinc. The relatively low melting point of brass and its fluidity make it a relatively easy material to cast. Brass can range in surface color from red to yellow to gold to silver depending on the zinc content. Some of the common uses for brass alloys include costume jewelry, locks, hinges, gears, bearings, hose couplings, ammunition casings, automotive radiators, musical instruments, electronic packaging, and coins.
Nickel Silver
Nickel silver, also known as German silver, nickel brass, or alpaca, is a copper alloy with nickel and often zinc. The usual formulation is 60% copper, 20% nickel, and 20% zinc. For example the alloy C75700 contains 63.5 to 66.5% of Cu, 11.0 to 13.0% of Ni, 0.05% Pb max, 0.25% Fe max, 0.5% Mn max, and balance of Zn. UNS C75700 nickel silver 65-12 copper alloy has good corrosion and tarnish resistance and high formability. Nickel silver is named due to its silvery appearance, but it contains no elemental silver unless plated. Nickel silver alloys are used for decorative applications, jewelry, model making, musical instruments (e.g.,, flutes, clarinets), flutes ballpoint refills, screws, rivets and fishing rods, and test probes.
Zamak – Zamak 3
Zamak is a family of alloys with a base metal of zinc and alloying elements of aluminium, magnesium, and copper. Alloys of zinc with small amounts of copper, aluminum, and magnesium are useful in die casting as well as spin casting, especially in the automotive, electrical, and hardware industries. Zinc alloys have low melting points, require relatively low heat input, and do not require fluxing or protective atmospheres. Because of their high fluidity, zinc alloys can be cast in much thinner walls than other die castings alloys, and they can be die cast to tighter dimensional tolerances. These zinc alloys are marketed under the name Zamak. The name Zamak is an acronym of the German names for the metals of which the alloys are composed: Zink (zinc), Aluminium, Magnesium, and Kupfer (copper). The low melting point and the low viscosity of the alloy make possible the production of small and intricate shapes.
For example, Zamak 3 (ASTM AG40A), or Zinc Alloy 3, is the most widely used zinc alloy in the zinc die casting industry and is usually the first choice when considering zinc for die casting for a number of reasons. It provides the best overall combination of strength, castability, dimensional stability, ease of finishing, and cost.
- Excellent physical and mechanical properties
- Excellent castability and long-term dimensional stability
- Excellent finishing characteristics for plating, painting, and chromate treatments
- Excellent damping capacity and vibration attenuation in comparison to aluminum die-cast alloys
Typical applications include die-castings such as automotive parts, household appliances and fixtures, office and computer equipment, and building hardware.
Properties of Zinc Alloys – Zamak 3
Material properties are intensive properties, which means they are independent of the amount of mass and may vary from place to place within the system at any moment. Materials science involves studying materials’ structure and relating them to their properties (mechanical, electrical, etc.). Once materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and, thus, its properties are its constituent chemical elements and the way it has been processed into its final form.
The density of Zinc Alloys – Zamak 3
Density of zinc alloy – Zamak 3 is 6.6 g/cm3 (0.24 lb/in3).
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
Since the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance, it is obvious, that the density of a substance strongly depends on its atomic mass and also on the atomic number density (N; atoms/cm3),
- Atomic Weight. The atomic mass is carried by the atomic nucleus, which occupies only about 10-12 of the atom’s total volume or less, but it contains all the positive charge and at least 99.95% of the atom’s total mass. Therefore it is determined by the mass number (number of protons and neutrons).
- Atomic Number Density. The atomic number density (N; atoms/cm3), which is associated with atomic radii, is the number of atoms of a given type per unit volume (V; cm3) of the material. The atomic number density (N; atoms/cm3) of a pure material having an atomic or molecular weight (M; grams/mol) and the material density (⍴; gram/cm3) is easily computed from the following equation using Avogadro’s number (NA = 6.022×1023 atoms or molecules per mole):
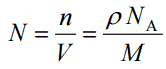
- Crystal Structure. The density of a crystalline substance is significantly affected by its crystal structure. FCC structure, along with its hexagonal relative (hcp), has the most efficient packing factor (74%). Metals containing FCC structures include austenite, aluminum, copper, lead, silver, gold, nickel, platinum, and thorium.
Mechanical Properties of Zinc Alloys – Zamak 3
Materials are frequently chosen for various applications because they have desirable combinations of mechanical characteristics. For structural applications, material properties are crucial, and engineers must take them into account.
Strength of Zinc Alloys – Zamak 3
In the mechanics of materials, the strength of a material is its ability to withstand an applied load without failure or plastic deformation. The strength of materials basically considers the relationship between the external loads applied to a material and the resulting deformation or change in material dimensions. The strength of a material is its ability to withstand this applied load without failure or plastic deformation.
Ultimate Tensile Strength
The ultimate tensile strength of zinc alloy – Zamak 3 is about 268 MPa.
 The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, the fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steels.
The ultimate tensile strength is the maximum on the engineering stress-strain curve. This corresponds to the maximum stress that can be sustained by a structure in tension. Ultimate tensile strength is often shortened to “tensile strength” or even to “the ultimate.” If this stress is applied and maintained, the fracture will result. Often, this value is significantly more than the yield stress (as much as 50 to 60 percent more than the yield for some types of metals). When a ductile material reaches its ultimate strength, it experiences necking where the cross-sectional area reduces locally. The stress-strain curve contains no higher stress than the ultimate strength. Even though deformations can continue to increase, the stress usually decreases after achieving the ultimate strength. It is an intensive property; therefore its value does not depend on the size of the test specimen. However, it is dependent on other factors, such as the preparation of the specimen, the presence or otherwise of surface defects, and the temperature of the test environment and material. Ultimate tensile strengths vary from 50 MPa for aluminum to as high as 3000 MPa for very high-strength steels.
Yield Strength
The yield strength of zinc alloy – Zamak 3 is about 208 MPa.
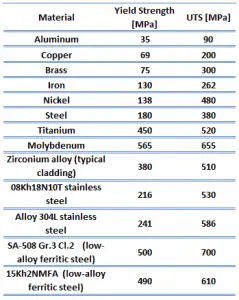
The yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning plastic behavior. Yield strength or yield stress is the material property defined as the stress at which a material begins to deform plastically. In contrast, the yield point is where nonlinear (elastic + plastic) deformation begins. Before the yield point, the material will deform elastically and return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible. Some steels and other materials exhibit a behavior termed a yield point phenomenon. Yield strengths vary from 35 MPa for low-strength aluminum to greater than 1400 MPa for very high-strength steel.
Young’s Modulus of Elasticity
Young’s modulus of elasticity of zinc alloy – Zamak 3 is about 96 GPa.
Young’s modulus of elasticity is the elastic modulus for tensile and compressive stress in the linear elasticity regime of a uniaxial deformation and is usually assessed by tensile tests. Up to limiting stress, a body will be able to recover its dimensions on the removal of the load. The applied stresses cause the atoms in a crystal to move from their equilibrium position. All the atoms are displaced the same amount and still maintain their relative geometry. When the stresses are removed, all the atoms return to their original positions, and no permanent deformation occurs. According to Hooke’s law, the stress is proportional to the strain (in the elastic region), and the slope is Young’s modulus. Young’s modulus is equal to the longitudinal stress divided by the strain.
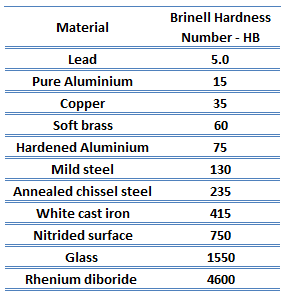
The hardness of Zinc Alloys – Zamak 3
Brinell hardness of zinc alloy – Zamak 3 is approximately 82 HB.
Rockwell hardness test is one of the most common indentation hardness tests, that has been developed for hardness testing. In contrast to the Brinell test, the Rockwell tester measures the depth of penetration of an indenter under a large load (major load) compared to the penetration made by a preload (minor load). The minor load establishes the zero position. The major load is applied and removed while maintaining the minor load. The difference between the depth of penetration before and after application of the major load is used to calculate the Rockwell hardness number. That is, the penetration depth and hardness are inversely proportional. The chief advantage of Rockwell hardness is its ability to display hardness values directly. The result is a dimensionless number noted as HRA, HRB, HRC, etc., where the last letter is the respective Rockwell scale.
The Rockwell C test is performed with a Brale penetrator (120°diamond cone) and a major load of 150kg.
Thermal Properties of Zinc Alloys – Zamak 3
Thermal properties of materials refer to the response of materials to changes in their temperature and the application of heat. As a solid absorbs energy in the form of heat, its temperature rises, and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are often critical in solids’ practical use.
Melting Point of Zinc Alloys – Zamak 3
The melting point of zinc alloy – Zamak 3 is around 385°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition in which the solid and liquid can exist in equilibrium.
Thermal Conductivity of Zinc Alloys – Zamak 3
The thermal conductivity of zinc alloy – Zamak 3 is 113 W/(m. K).
The heat transfer characteristics of solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It is a measure of a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies to all matter, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it also depends upon pressure. In general:
Most materials are very nearly homogeneous, therefore, we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.