 Corrosion is the deterioration of a material due to chemical interaction with its environment. It is a natural process in which metals convert their structure into a more chemically-stable form, such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon since it is associated with the mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even noble metals, such as gold, are subject to corrosive attack in some environments.
Corrosion is the deterioration of a material due to chemical interaction with its environment. It is a natural process in which metals convert their structure into a more chemically-stable form, such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon since it is associated with the mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion. Even noble metals, such as gold, are subject to corrosive attack in some environments.
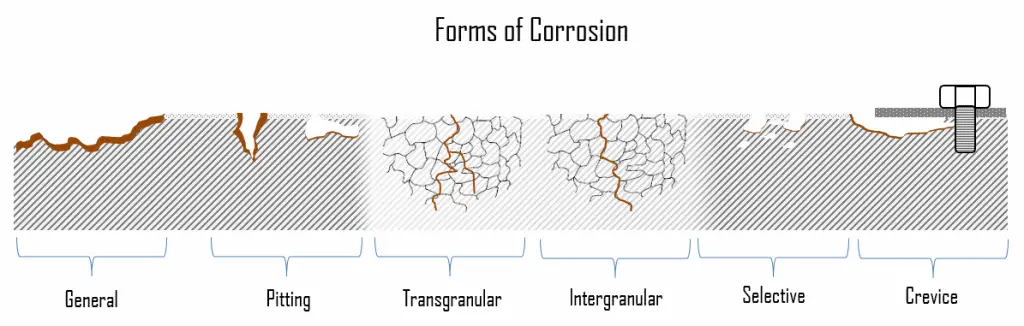
Forms of Corrosion
The areas over which the anodic and cathodic reactions occur can vary greatly during metal corrosion. Corrosion can come in different forms and grow at different rates. This results in various forms of corrosion, such as a uniform attack, pitting, and crevice corrosion. The problem is that many forms of corrosion exist, and each is caused by different reasons and undergoes different mechanisms. Moreover, each form of corrosion has its special mechanism, which can be quite complex in some cases. This is especially problematic when two or more corrosion types occur simultaneously.
In the following section, we will briefly describe the most common forms of corrosion. They are divided into two subcategories: general (uniform) and localized form of corrosion.
- General Corrosion. General corrosion, also known as uniform corrosion, is a form of corrosion that affects the entire surface of the metal, whereas other forms affect a specific spot or portion. This type of corrosion is commonly observed in pure metals, which are metallurgical and compositionally uniform. It is a very slow reaction that is fairly evenly distributed over the entire metal surface exposed to any circulating water. The fact that it affects a fairly large area of the metal makes it much easier to detect and hence much less severe than localized corrosion. The problem with general corrosion is that it results in a large volume of oxides that tend to attach themselves to the heat transfer surfaces and affect the system’s efficiency.
- Localized Corrosion. In localized corrosion, there is an intense attack at localized sites on the surface of a component while the rest of the surface is corroding at a much lower rate. Localized corrosion occurs when corrosion works with other destructive processes such as stress, fatigue, erosion, and other forms of a chemical attack.
General Corrosion
General corrosion, also known as uniform corrosion, is a form of corrosion that affects the entire surface of the metal, whereas other forms affect a specific spot or portion. It is the most common form of corrosion. This type of corrosion is commonly observed in pure metals, which are metallurgical and compositionally uniform. Weathering steels, magnesium alloys, zinc alloys, and copper alloys are materials typically exhibiting general corrosion. Passive materials, such as stainless steel, aluminum alloys, or nickel-chromium alloys, are generally subject to localized corrosion.
It is a very slow reaction that is fairly evenly distributed over the entire metal surface exposed to any circulating water. The fact that it affects a fairly large area of the metal makes it much easier to detect and hence much less severe than localized corrosion. The problem with general corrosion is that it results in a large volume of oxides that tend to attach themselves to the heat transfer surfaces and affect the system’s efficiency.
General Corrosion – Protection
Some standard methods associated with material selection that protect against general corrosion include:
- Using corrosion-resistant materials such as stainless steel and nickel, chromium, and molybdenum alloys.
- The use of protective coatings such as paints and epoxies.
- The application of metallic and nonmetallic coatings or linings to the surface protects against corrosion but allows the material to retain its structural strength (for example, a carbon steel pressure vessel with stainless steel cladding as a liner).
Localized Corrosion
In localized corrosion, there is an intense attack at localized sites on the surface of a component while the rest of the surface is corroding at a much lower rate. Localized corrosion occurs when corrosion works with other destructive processes such as stress, fatigue, erosion, and other forms of a chemical attack. Localized corrosion mechanisms can cause more damage than any one of those destructive processes individually. Localized corrosion can further be classified as pitting corrosion, galvanic corrosion, crevice corrosion, selective corrosion, erosion corrosion, intergranular corrosion, chloride stress corrosion, and stress corrosion cracking (SCC). Passive materials, such as stainless steel, aluminum alloys, or nickel-chromium alloys, are generally subject to localized corrosion.
Pitting Corrosion
Pitting, characterized by sharply defined holes, is one of the most insidious forms of corrosion. They ordinarily penetrate from the top of a horizontal surface downward in a nearly vertical direction, and it is supposed that gravity causes the pits to grow downward. Pitting corrosion can cause failure by perforation while producing only a small weight loss on the metal. This perforation can be difficult to detect, and its growth is rapid, leading to unexpected loss of function of the component. Pitting corrosion has also been associated with both crevice and galvanic corrosion. Metal deposition (copper ions plated on a steel surface) can also create sites for pitting attacks.
Causes of pitting corrosion include:
- Local inhomogeneity on the metal surface
- Local loss of passivity
- Mechanical or chemical rupture of a protective oxide coating
- Galvanic corrosion from a relatively distant cathode
With corrosion-resistant alloys, such as stainless steel, the most common cause of pitting corrosion is the highly localized destruction of passivity by contact with moisture that contains halide ions, particularly chlorides. However, alloying with about 2% molybdenum enhances their resistance significantly. Chloride-induced pitting of stainless steels usually results in undercutting, producing enlarged subsurface cavities or caverns.
Pitting Corrosion – Protection
Pitting corrosion is a hazard due to the possible rapid penetration of the metal with little overall loss of mass. Pitting corrosion is minimized by:
- Avoiding stagnant conditions
- Using the correct metals and alloys that are less susceptible to the corrosion
- Avoiding agents in the medium that cause pitting
- Designing the system and components such that no crevices are present
Crevice Corrosion
Crevice corrosion refers to the localized corrosion at the crevice or gap between two or more joining metals. Crevice corrosion is a pitting corrosion that occurs specifically within the low flow region of a crevice. This type of attack is usually associated with small volumes of stagnant solution caused by holes, gaskets surface, lap joints, surface deposits, and crevices under bolt and rivets heads. The damage occurs due to the difference in constituents’ concentration, mainly oxygen, on the surfaces involved. Crevice corrosion can progress rapidly (tens to hundreds of times faster than the normal rate of general corrosion in the same given solution).
Crevice corrosion is a hazard due to the possible rapid penetration of the metal with little overall loss of mass. Crevice corrosion is minimized by:
- Crevice corrosion may be prevented by using welded instead of riveted or bolted joints
- Using the correct metals and alloys that are less susceptible to the corrosion
- Avoiding agents in the medium that cause pitting
- Designing the system and components such that no crevices are present
Galvanic Corrosion
Galvanic corrosion occurs when two dissimilar metals are immersed in a conductive solution in presence of some potential difference, and there is a flow of electrons between the metals. It may also occur with one metal with heterogeneities (dissimilarities) (for example, impurity inclusions, grains of different sizes, differences in composition of grains, or differences in mechanical stress). The metal which is less corrosive resistant becomes anode, and the metal with more corrosive resistance becomes a cathode. The corrosion of the less corrosive resistance is usually increased, and attack on more resistant material is decreased. A difference in electrical potential exists between the different metals and serves as the driving force for electrical current flow through the corroded or electrolyte.
Galvanic corrosion occurs only if the following conditions are met:
- Two different metals must be present.
- The two metals must be in contact, or an electrically conductive path between the two must be present.
- There must be an electrically conductive path for the ions to move from the “anode” to the “cathode.”
If any of these conditions are not satisfied, galvanic corrosion will not likely occur.
Galvanic corrosion only causes the deterioration of one of the metals. The stronger, more noble one is cathodic (positive) and protected. This is the mechanism of galvanic anodes, which are the main component of a galvanic cathodic protection (CP) system used to protect buried or submerged metal structures from corrosion. In some instances, galvanic corrosion can be helpful.
Selective Leaching – Selective Corrosion
Selective leaching or selective corrosion removes one element from a solid alloy by the corrosion process. The most common example is the dezincification of brass, in which zinc is selectively leached from a copper-zinc brass alloy, and this process produces a weakened porous copper structure. The selective removal of zinc can be in a uniform manner or localized scale.
However, many alloys are subject to selective leaching under certain conditions. A similar process occurs in other alloy systems in which aluminum, iron, cobalt, chromium, and other elements are removed. Elements in an alloy that are more resistant to the environment remain behind. Two mechanisms are involved:
- Two metals in an alloy are dissolved; one metal redeposits on the surface of the surviving elements.
- One metal is selectively dissolved, leaving the other metals behind.
The first system involves the dezincification of brasses, and the second system is involved when molybdenum is removed from nickel alloys in molten sodium hydroxide.
Erosion Corrosion
Erosion corrosion is the cumulative damage induced by electrochemical corrosion reactions and mechanical effects from relative motion between the electrolyte and the corroding surface. Erosion can also occur in combination with other forms of degradation, such as corrosion, and this is referred to as erosion-corrosion. Erosion corrosion is a material degradation process due to the combined effect of corrosion and wear. Nearly all flowing or turbulent corrosive media can cause erosion corrosion. The mechanism can be described as follows:
- mechanical erosion of the material, or protective (or passive) oxide layer on its surface,
- enhanced corrosion of the material if the corrosion rate of the material depends on the thickness of the oxide layer.
Erosion corrosion is found in the systems such as piping, valves, pumps, nozzles, heat exchangers, and turbines. Wear is a mechanical material degradation process occurring on rubbing or impacting surfaces, while corrosion involves chemical or electrochemical reactions of the material. Corrosion may accelerate wear, and wear may accelerate corrosion.
Intergranular Corrosion – Weld Decay
Intergranular corrosion (IGC) is preferential corrosion along the grain boundaries of material. For some alloys and in specific environments. This type of corrosion is especially prevalent in some stainless steel. In stainless steel, intergranular corrosion may occur due to the precipitation of chromium carbides (Cr23C6) or intermetallic phases.
The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below is impossible. But the chromium carbides may precipitate in the grain boundaries, which results in depletion of chromium in the zones close to the grain boundaries due to the diffusion rate of chromium that is slow. The chromium-depleted zones become less corrosion-resistant than the rest of the matrix. In a corrosive environment, the depleted areas may be activated, and corrosion will occur in narrow areas between the grains.
Intergranular corrosion is an especially severe problem in the welding of stainless steel. When it is often termed weld decay. Also, stainless steel, which has been heat-treated in a way that produces grain boundary precipitates and adjacent chromium depleted zones, is sensitized. Stainless steels can be stabilized against this behavior by the addition of titanium, niobium, or tantalum, which form titanium carbide, niobium carbide, and tantalum carbide preferentially to chromium carbide, by lowering the content of carbon in the steel and case of welding also in the filler metal under 0.02%, or by heating the entire part above 1000 °C and quenching it in water, leading to the dissolution of the chromium carbide in the grains and then preventing its precipitation.
There are two special cases of intergranular corrosion, but these mechanisms are treated separately:
- Stress corrosion cracking. Intergranular corrosion induced by environmental stresses is termed stress corrosion cracking.
- Chloride stress corrosion cracking. Intergranular corrosion induced by the combined action of environmental stresses and chlorine is termed chloride stress corrosion cracking.
Stress Corrosion Cracking – SCC
One of the most serious metallurgical problems and a major concern in the nuclear industry is stress-corrosion cracking (SCC). Stress-corrosion cracking results from the combined action of applied tensile stress and a corrosive environment. Both influences are necessary. SCC is a type of intergranular attack corrosion that occurs at the grain boundaries under tensile stress. It tends to propagate as stress opens cracks subject to corrosion, which is then corroded further, weakening the metal by further cracking. The cracks can follow intergranular or transgranular paths, and there is often a tendency for crack branching. Failure behavior is characteristic of that brittle material, even though the metal alloy is intrinsically ductile. SCC can lead to unexpected sudden failure of normally ductile metal alloys subjected to tensile stress, especially at elevated temperatures. SCC is highly chemically specific in that certain alloys are likely to undergo SCC only when exposed to a small number of chemical environments.
See also: Stress Corrosion Cracking.
The most effective means of preventing SCC in reactor systems are:
- designing properly
- reducing stress
- removing critical environmental species such as hydroxides, chlorides, and oxygen
- avoiding stagnant areas and crevices in heat exchangers where chloride and hydroxide might become concentrated.
Chloride Stress Corrosion Cracking
Chloride stress corrosion occurs in austenitic stainless steels under tensile stress in the presence of oxygen, chloride ions, and high temperature. It is one of the most important forms of stress corrosion that concerns the nuclear industry. Austenitic stainless steels contain between 16 and 25% Cr and can also contain nitrogen in solution, which contribute to their relatively high uniform corrosion resistance. One type of corrosion which can attack austenitic stainless steel is chloride stress corrosion.
The three conditions that must be present for chloride stress corrosion to occur are as follows:
- Chloride ions are present in the environment
- Dissolved oxygen is present in the environment
- Metal is under tensile stress
Chloride stress corrosion involves the selective attack of the metal along grain boundaries. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below is impossible. But the chromium carbides may precipitate in the grain boundaries, which results in depletion of chromium in the zones close to the grain boundaries due to the diffusion rate of chromium that is slow. The chromium-depleted zones become less corrosion-resistant than the rest of the matrix. In a corrosive environment, the depleted areas may be activated, and corrosion will occur in narrow areas between the grains.
It has been found that this is closely associated with certain heat treatments resulting from welding. This can be minimized considerably by proper annealing processes. This form of corrosion is controlled by maintaining low chloride ion and oxygen content in the environment and the use of low carbon steels. Ferritic stainless steels are chosen for their resistance to stress corrosion cracking, which makes them an attractive alternative to austenitic stainless steels in applications where chloride-induced SCC is prevalent.