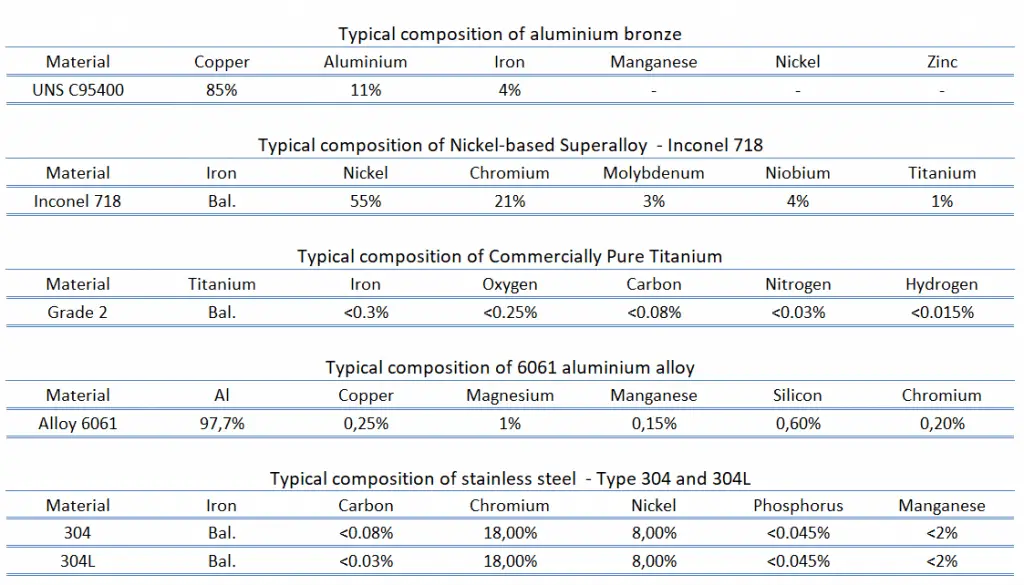
Corrosion is the deterioration of a material due to chemical interaction with its environment. It is a natural process in which metals convert their structure into a more chemically-stable form, such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon since it is associated with the mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion, and even noble metals, such as gold, are subject to corrosive attack in some environments.
Corrosion is the deterioration of a material due to chemical interaction with its environment. It is a natural process in which metals convert their structure into a more chemically-stable form, such as oxides, hydroxides, or sulfides. The consequences of corrosion are all too common. Familiar examples include the rusting of automotive body panels and pipings and many tools. Corrosion is usually a negative phenomenon since it is associated with the mechanical failure of an object. Metal atoms are removed from a structural element until it fails, or oxides build up inside a pipe until it is plugged. All metals and alloys are subject to corrosion, and even noble metals, such as gold, are subject to corrosive attack in some environments.
Most metals are not thermodynamically stable in the metallic form; they want to corrode and revert to the more stable forms normally found in ores, such as oxides. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although the term “degradation” is more common in this context in this context. Ceramic materials are relatively resistant to deterioration, usually at elevated temperatures or in extreme environments, and the process is frequently called corrosion. For polymers, mechanisms and consequences differ from those for metals and ceramics, and degradation is most frequently used. Corrosion degrades the useful properties of materials and structures, including strength, appearance, and permeability to liquids and gases.
Corrosion is electrochemical because corrosive chemical reactions involve a transfer of charge. The corrosion chemistry is quite complex, but it may be considered an electrochemical phenomenon. The metal ions enter the solution, causing the metal to become negatively charged with the electrolyte. The difference in the charge causes a potential to develop and produces a voltage between the electrolyte and the metal.
Corrosion, as a natural and persistent process, also involves an unintended deterioration of metals, sometimes with disastrous outcomes. How big is the corrosion problem? The problem of metallic corrosion is significant. In economic terms, it has been estimated that approximately 5% of an industrialized nation’s income is spent on corrosion prevention and the maintenance or replacement of products lost or contaminated due to corrosion reactions.
Corrosion is of primary concern in nuclear reactor plants. Corrosion occurs continuously throughout the reactor plant, and every metal is subject to it. Even though this corrosion cannot be eliminated, it can be controlled.
Protection from Corrosion
As was written, the problem of metallic corrosion is significant. In economic terms, it has been estimated that approximately 5% of an industrialized nation’s income is spent on corrosion prevention and the maintenance or replacement of products lost or contaminated due to corrosion reactions. Therefore, various treatments slow corrosion damage to metallic objects exposed to the weather, salt water, acids, or other hostile environments. Since there are many forms of corrosion, there are many ways to stop or mitigate corrosion. In every case, it depends on the material to be protected and the environment in which the material is used. Metals may be protected from corrosion by using a metal in an environment in which it is immune, making a physical barrier between the metal and its environment, using an electric current, or changing the environment.
- Material Selection. Perhaps the most common and easiest way of preventing corrosion is through the judicious selection of materials once the corrosion environment has been characterized. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environments and other conditions. Here, the cost may be a significant factor, and employing the material that provides the optimum corrosion resistance is not always economically feasible.
- Anti-corrosion Surface Treatment. A coating provides protection by forming a physical barrier between the metallic substrate and an aqueous corrosive environment. Coatings protect metallic structures from corrosion by both inhibition and barrier effects. The barrier effect depends on the adhesion to the under-layer but also the non-conducting properties of the coating. Penetration of water or ions is a major cause of loss of the barrier, which may lead to delamination of the coating and under-film corrosion. Plating, painting, and the application of enamel are the most common anti-corrosion treatments.
- Anodization. Anodization is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. A limited number of metals, such as stainless steel, can achieve passivity. The process is called anodizing because the part to be treated forms the anode electrode of an electrolytic cell. Aluminum alloys are usually anodized to increase corrosion resistance and allow dyeing (coloring).
- Cathodic Protection. Cathodic protection is a very effective way of protection from corrosion based on using a more anodic material than metal to be protected. The protected metal is electrically connected to another metal, a more reactive metal in the particular environment. The metal which is less corrosive resistant becomes an anode, and the metal with more corrosive resistance becomes a cathode. The corrosion of the less corrosive resistance is usually increased, and the attack on the more resistant material is decreased. A difference in electrical potential exists between the different metals and serves as the driving force for electrical current flow through the corrodant or electrolyte. Cathodic protection systems such as steel pipelines and tanks, such as steel pipelines and tanks, are most commonly used to protect buried or submerged metal structures from corrosion. Magnesium, zinc, and aluminum alloys are common sacrificial anodes. Magnesium anodes are most commonly used for buried soil applications. Zinc is most often used for freshwater and saltwater marine applications. Aluminum alloys are most often used for offshore structures.
- Corrosion Inhibitors. If the environment is controlled (especially in recirculating systems), corrosion inhibitors can often be added to it. These chemicals form an electrically insulating or chemically impermeable coating on exposed metal surfaces to suppress electrochemical reactions. Corrosion inhibitors are chemicals that, when added in relatively low concentrations to the environment, reduce the rate of a corrosive process. The substance acts like an inhibitor depending on the corrosive environment and the alloy. Inhibitors are normally used in closed systems such as automobile radiators and steam boilers. An example of this principle is the use of antifreeze in cars. The effectiveness of an inhibitor depends on several different mechanisms. Some react with the chemically active species in the solution, while others react with the corroding surface, interfering with the corrosive reaction or forming a thin protective coating. For example, reductive inhibitors such as amines and hydrazines generally remove oxygen. In this example, hydrazine converts oxygen, a common corrosive agent, to water, which is generally benign. Many inhibitors are also toxic and are, therefore, unsuitable for all applications. Another limitation of inhibitors is that they generally lose effectiveness when the temperature and concentration of the environment increase.
Corrosion-resistant Alloys
Corrosion-resistant alloys, as their name indicates, are alloys with enhanced corrosion resistance. Some ferrous and many non-ferrous metals and alloys are widely used in corrosive environments. In all cases, it strongly depends on certain environments and other conditions. Corrosion-resistant alloys are used for water piping and many chemical and industrial applications. In the case of ferrous alloys, we are talking about stainless steel and, to some extent about cast irons. But some non-ferrous corrosion-resistant alloys exhibit remarkable corrosion resistance; therefore, they may be used for many special purposes. There are two main reasons why non-ferrous materials are preferred over steel and stainless steels for many of these applications. For example, many of the non-ferrous metals and alloys possess much higher resistance to corrosion than available alloy steels and stainless steel grades. Second, a high strength-to-weight ratio or high thermal and electrical conductivity may provide a distinct advantage over a ferrous alloy.
Passivation
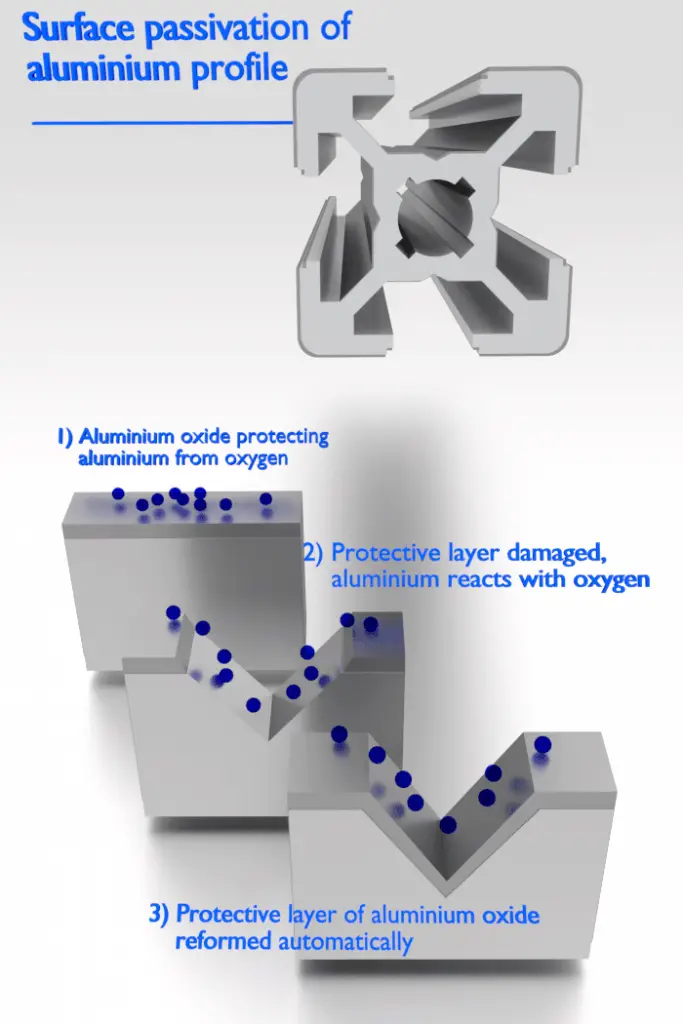 There are metals, that exhibit a passivity to corrosion. Passivity is the characteristic of a metal exhibited when that metal does not become active in the corrosion reaction. Passivation is a natural process of the buildup of a stable, tenacious layer of metal oxide or protective barrier on the surface of the metal that acts as a barrier separating the metal surface from the environment. Passivity decreases or stops the corrosion process because of the formation of the layer. Fortunately, from an engineering standpoint, the metals most susceptible to this kind of behavior are the common engineering and structural materials, including iron, nickel, silicon, chromium, titanium, and alloys containing these metals.
There are metals, that exhibit a passivity to corrosion. Passivity is the characteristic of a metal exhibited when that metal does not become active in the corrosion reaction. Passivation is a natural process of the buildup of a stable, tenacious layer of metal oxide or protective barrier on the surface of the metal that acts as a barrier separating the metal surface from the environment. Passivity decreases or stops the corrosion process because of the formation of the layer. Fortunately, from an engineering standpoint, the metals most susceptible to this kind of behavior are the common engineering and structural materials, including iron, nickel, silicon, chromium, titanium, and alloys containing these metals.
For example, stainless steel owes its corrosion-resistant properties to the formation of a self-healing passive surface film. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below is impossible. Once the surface is cleaned and the bulk composition of the stainless steel is exposed to air, the passive film forms immediately.
Aluminium is highly corrosion resistant in many environments because it also passivates. If damaged, the protective film normally re-forms very rapidly. However, a change in the character of the environment (e.g., alteration in the concentration of the active corrosive species) may cause a passivated material to revert to an active state. Generally, at high temperatures (in water, corrosion limits the use of aluminium to temperatures near 100°C), aluminum’s relatively low strength and poor corrosion properties make it unsuitable as a structural material.