Hardening of Metals
In materials science, hardness is the ability to withstand surface indentation (localized plastic deformation) and scratching. Hardness is probably the most poorly defined material property because it may indicate resistance to scratching, abrasion, an indentation, or even resistance to shaping or localized plastic deformation. Hardness is important from an engineering standpoint because resistance to wear by either friction or erosion by steam, oil, and water generally increases with hardness.
Hardening is a metallurgical metalworking process used to increase the hardness of a metal. The hardness of a metal is directly proportional to the uniaxial yield stress at the location of the imposed strain. To improve the hardness of the pure metal, we can use different ways, which include:
- Hall-Petch Method
- Solid Solution Hardening (alloying)
- Work Hardening (Cold Working)
- Precipitation Hardening
- Transformation hardening
- Dispersion Hardening
- Surface Hardening
Transformation Hardening
 Transformation hardening, also known as martensitic transformation hardening, is one of the most common hardening methods primarily used for steels (i.e., carbon steels and stainless steels). The martensitic transformation is not unique to iron-carbon al and is found in other systems and is characterized, in part, by diffusionless transformation.
Transformation hardening, also known as martensitic transformation hardening, is one of the most common hardening methods primarily used for steels (i.e., carbon steels and stainless steels). The martensitic transformation is not unique to iron-carbon al and is found in other systems and is characterized, in part, by diffusionless transformation.
Martensitic steels use predominantly higher levels of C and Mn and heat treatment to increase strength. The finished product will have a duplex ferrite microstructure with varying levels of degenerate martensite, allowing for varying levels of strength. In metallurgy, quenching is commonly used to harden steel by introducing martensite. There is a balance between hardness and toughness in any steel; the harder the steel, the less tough or impact-resistant it is, and the more impact-resistant it is, the less hard it is.
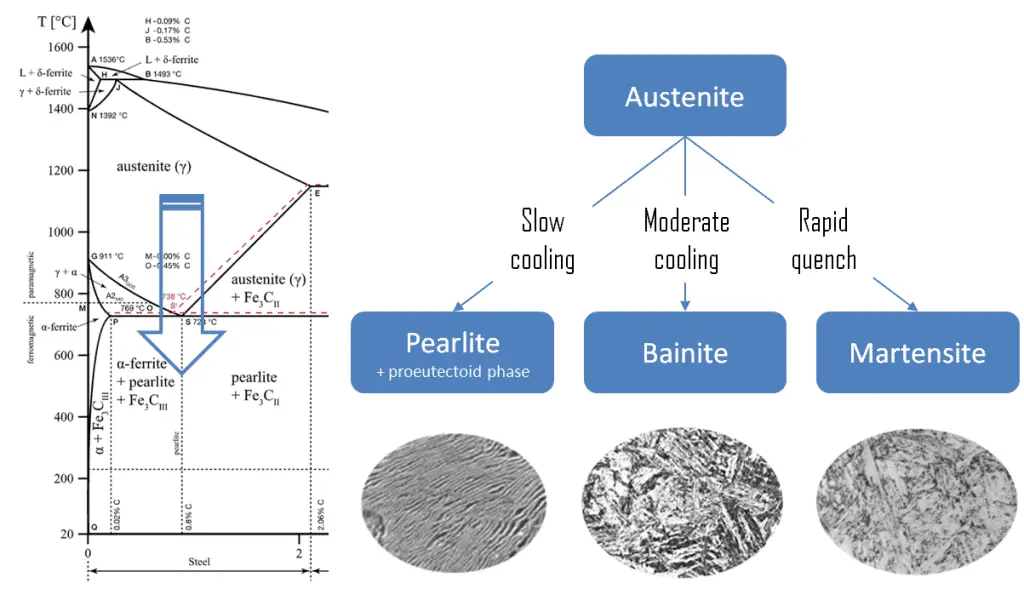
Martensite is produced from austenite due to quenching or another form of rapid cooling. Austenite in iron-carbon alloys is generally only present above the critical eutectoid temperature (723°C) and below 1500°C, depending on carbon content. In the case of normal cooling rates, as the austenite cools, the carbon diffuses out of the austenite, forms carbon-rich iron-carbide (cementite), and leaves behind carbon-poor ferrite. Depending on alloy composition, a layering of ferrite and cementite, called pearlite, may form. But in case of rapid cooling, the carbon does not have time enough to diffuse and then transforms into a highly strained body-centered tetragonal form called martensite that is supersaturated with carbon. All the carbon atoms remain interstitial impurities in martensite. The cooling rate determines the relative proportions of martensite, ferrite, and cementite. It, therefore, determines the mechanical properties of the resulting steel, such as hardness, tensile strength, and toughness.
Tempered Martensite
The relative ability of a ferrous alloy to form martensite is called hardenability. Hardenability is commonly measured as the distance below a quenched surface at which the metal exhibits a specific hardness of 50 HRC, for example, or a specific percentage of martensite in the microstructure. The highest hardness of pearlitic steel is 43 HRC, whereas martensite can achieve 72 HRC. Fresh martensite is very brittle if the carbon content is greater than approximately 0.2 to 0.3%. It is so brittle that it cannot be used for most applications. This brittleness can be removed (with some loss of hardness) if the quenched steel is heated slightly in a process known as tempering.