 In metallurgy, stainless steel is a steel alloy with at least 10.5% chromium with or without other alloying elements and a maximum of 1.2% carbon by mass. Stainless steels, also known as inox steels or inox from French inoxydable (inoxidizable), are steel alloys that are very well known for their corrosion resistance, which increases with increasing chromium content. Corrosion resistance may also be enhanced by nickel and molybdenum additions. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below is impossible. Chromium can be used as a hardening element and is frequently used with a toughening element such as nickel to produce superior mechanical properties.
In metallurgy, stainless steel is a steel alloy with at least 10.5% chromium with or without other alloying elements and a maximum of 1.2% carbon by mass. Stainless steels, also known as inox steels or inox from French inoxydable (inoxidizable), are steel alloys that are very well known for their corrosion resistance, which increases with increasing chromium content. Corrosion resistance may also be enhanced by nickel and molybdenum additions. The resistance of these metallic alloys to the chemical effects of corrosive agents is based on passivation. For passivation to occur and remain stable, the Fe-Cr alloy must have a minimum chromium content of about 10.5% by weight, above which passivity can occur and below is impossible. Chromium can be used as a hardening element and is frequently used with a toughening element such as nickel to produce superior mechanical properties.
Types of Stainless Steels
Stainless steel is a generic term for a large family of corrosion-resistant alloys containing at least 10.5% chromium and may contain other alloying elements. Numerous grades of stainless steel have varying chromium and molybdenum contents and varying crystallographic structures to suit the environment the alloy must endure. Stainless steels can be divided into five categories:
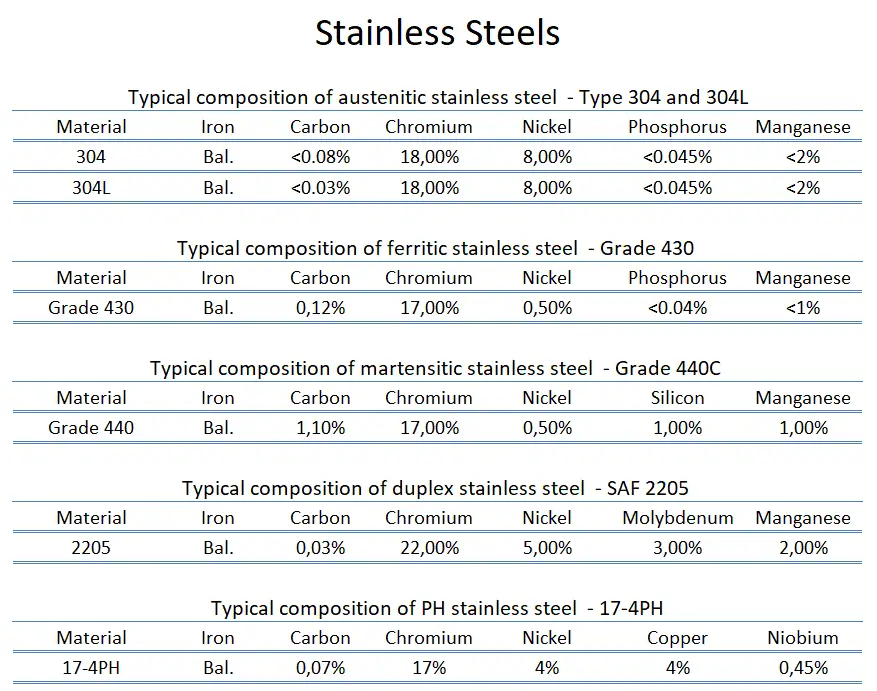 Ferritic stainless steel. In ferritic stainless steels, carbon is kept to low levels (C<0.08%), and the chromium content can range from 10.50 to 30.00%. They are called ferritic alloys because they contain primarily ferritic microstructures at all temperatures and cannot be hardened through heat treating and quenching. They are classified with AISI 400-series designations. While some ferritic grades contain molybdenum (up to 4.00%), only chromium is present as the main metallic alloying element. They are usually limited to relatively thin sections due to a lack of toughness in welds. Moreover, they have relatively poor high-temperature strength. Ferritic steels are chosen for their resistance to stress corrosion cracking, which makes them an attractive alternative to austenitic stainless steels in applications where chloride-induced SCC is prevalent.
Ferritic stainless steel. In ferritic stainless steels, carbon is kept to low levels (C<0.08%), and the chromium content can range from 10.50 to 30.00%. They are called ferritic alloys because they contain primarily ferritic microstructures at all temperatures and cannot be hardened through heat treating and quenching. They are classified with AISI 400-series designations. While some ferritic grades contain molybdenum (up to 4.00%), only chromium is present as the main metallic alloying element. They are usually limited to relatively thin sections due to a lack of toughness in welds. Moreover, they have relatively poor high-temperature strength. Ferritic steels are chosen for their resistance to stress corrosion cracking, which makes them an attractive alternative to austenitic stainless steels in applications where chloride-induced SCC is prevalent.- Austenitic stainless steel. Austenitic stainless steels contain between 16 and 25% Cr and can also contain nitrogen in solution, which contributes to their relatively high corrosion resistance. They are classified with AISI 200- or 300-series designations; the 300-series grades are chromium-nickel alloys, and the 200-series represent a set of compositions in which manganese and/or nitrogen replace some of the nickel. Austenitic stainless steels have the best corrosion resistance of all stainless steels, and they have excellent cryogenic properties and good high-temperature strength. They possess a nonmagnetic face-centered cubic (fcc) microstructure that can be easily welded. This austenite crystalline structure is achieved by sufficient additions of the austenite stabilizing elements nickel, manganese, and nitrogen. Austenitic stainless steel is the largest family of stainless steel, making up two-thirds of all stainless steel production. Their yield strength is low (200 to 300MPa), which limits their use for structural and other load-bearing components. They cannot be hardened by heat treatment but have the useful property of being work hardened to high strength levels while retaining a useful level of ductility and toughness. Duplex stainless steels tend to be preferred in such situations because of their high strength and corrosion resistance. The best-known grade is AISI 304 stainless, which contains chromium (between 15% and 20%) and nickel (between 2% and 10.5%) metals as the main non-iron constituents. 304 stainless steel has excellent resistance to a wide range of atmospheric environments and many corrosive media. Cold forming usually characterizes these alloys as ductile, weldable, and hardenable.
- Martensitic stainless steel. Martensitic stainless steels are similar to ferritic steels based on chromium but have higher carbon levels, as high as 1%. They are sometimes classified as low-carbon and high-carbon martensitic stainless steel. They contain 12 to 14% chromium, 0.2 to 1% molybdenum, and no significant amount of nickel. Higher amounts of carbon allow them to be hardened and tempered, much like carbon and low-alloy steels. They have moderate corrosion resistance but are considered hard, strong, and slightly brittle. They are magnetic and can be nondestructively tested using the magnetic particle inspection method, unlike austenitic stainless steel. A common martensitic stainless is AISI 440C, which contains 16 to 18% chromium and 0.95 to 1.2% carbon. Grade 440C stainless steel is used in the following applications: gage blocks, cutlery, ball bearings and races, molds and dies, and knives. As was written, martensitic stainless steels can be hardened and tempered through multiple ways of aging/heat treatment: The metallurgical mechanisms responsible for the martensitic transformations that take place in these stainless alloys during austenitizing and quenching are essentially the same as those that are used to harden lower-alloy-content carbon and alloy steels. The heat treatment typically involves three steps:
 Austenitizing, in which the steel is heated to a temperature in the range of 980 – 1050 °C depending on the grades. The austenite is a face-centered cubic phase.
Austenitizing, in which the steel is heated to a temperature in the range of 980 – 1050 °C depending on the grades. The austenite is a face-centered cubic phase.- Quenching. After austenitizing, the steels must be quenched. Martensitic stainless alloys can be quenched using still air, positive pressure vacuum, or interrupted oil quenching. The austenite is transformed into martensite, a hard body-centered tetragonal crystal structure. The martensite is very hard and too brittle for most applications.
- Tempering, i.e., heating to around 500 °C, holding at temperature, then air cooling. Increasing the tempering temperature decreases the yield strength and ultimate tensile strength but increases the elongation and the impact resistance.
- Duplex Stainless Steels. As their name indicates, Duplex stainless steels combine two main alloy types. They have a mixed microstructure of austenite and ferrite, the aim usually being to produce a 50/50 mix, although, in commercial alloys, the ratio may be 40/60. Their corrosion resistance is similar to their austenitic counterparts. Still, their stress-corrosion resistance (especially to chloride stress corrosion cracking), tensile strength, and yield strengths (roughly twice the yield strength of austenitic stainless steels) are generally superior to that of the austenitic grades. In duplex stainless steel, carbon is kept to very low levels (C<0.03%). Chromium content ranges from 21.00 to 26.00%, nickel content ranges from 3.50 to 8.00%, and these alloys may contain molybdenum (up to 4.50%). Toughness and ductility generally fall between those of the austenitic and ferritic grades. Duplex grades are usually divided into three sub-groups based on their corrosion resistance: lean duplex, standard duplex, and super duplex. Super duplex steels have enhanced strength and resistance to all forms of corrosion compared to standard austenitic steels. Common uses include marine applications, petrochemical plants, desalination plants, heat exchangers, and the papermaking industry. Today, the oil and gas industry is the largest user and has pushed for more corrosion-resistant grades, leading to the development of super duplex steels.
- PH Stainless Steels. PH stainless steels (precipitation-hardening) contain around 17% chromium and 4% nickel. These steels can develop very high strength through additions of aluminum, titanium, niobium, vanadium, and/or nitrogen, which form coherent intermetallic precipitates during a heat treatment process referred to as heat aging. As the coherent precipitates form throughout the microstructure, they strain the crystalline lattice and impede the movement of dislocations or defects in a crystal’s lattice. Since dislocations are often the dominant carriers of plasticity, this hardens the material. For example, precipitation-hardened stainless steel 17-4 PH (AISI 630) has an initial microstructure of austenite or martensite. Austenitic grades are converted to martensitic grades through heat treatment (e.g., heat treatment at about 1040 °C followed by quenching) before precipitation hardening. Subsequent aging treatment at about 475 °C precipitates Nb and Cu-rich phases, increasing the strength to above 1000 MPa yield strength. Unlike austenitic alloys, heat treatment strengthens PH steel to levels higher than martensitic alloys. Precipitation-hardening stainless steels are designated by the AISI 600-series. Of all the available stainless grades, they generally offer the greatest combination of high strength and excellent toughness and corrosion resistance. They are as corrosion-resistant as austenitic grades. Common uses are in the aerospace and some other high-technology industries.