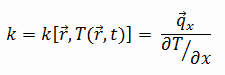Carbon steels are iron-carbon alloys that may contain appreciable concentrations of other alloying elements. Plain carbon steels are iron-carbon alloys in which the properties are primarily derived from the presence of carbon. Some incidental elements like manganese, silicon, sulfur, and phosphorus are present in small amounts due to the method of making steels and not modifying the mechanical properties. Adding a small amount of non-metallic carbon to iron trades its great ductility for greater strength. Due to its very-high strength but still substantial toughness, and its ability to be greatly altered by heat treatment, steel is one of the most useful and common ferrous alloys in modern use. Thousands of alloys have different compositions and/or heat treatments. The mechanical properties are sensitive to the content of carbon, which is normally less than 1.0 wt%.
Thermal Properties of Carbon Steel
Thermal properties of materials refer to the response of materials to changes in their temperature and the application of heat. As a solid absorbs energy in the form of heat, its temperature rises, and its dimensions increase. But different materials react to the application of heat differently.
Heat capacity, thermal expansion, and thermal conductivity are often critical in solids’ practical use.
Melting Point of Carbon Steel
The melting point of low-carbon steel is around 1450°C.
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting point also defines a condition where the solid and liquid can exist in equilibrium.
Thermal Conductivity of Carbon Steel
Low-carbon steel is a multi-element substance, mainly iron, with additions of carbon and impurities. The thermal conductivity of wrought iron is around 50 W/(m. K).
The heat transfer characteristics of solid material are measured by a property called the thermal conductivity, k (or λ), measured in W/m.K. It measures a substance’s ability to transfer heat through a material by conduction. Note that Fourier’s law applies to all matter, regardless of its state (solid, liquid, or gas). Therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature, and for vapors, it also depends upon pressure. In general:
Most materials are nearly homogeneous. Therefore we can usually write k = k (T). Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic material, the thermal conductivity is independent of the direction of transfer, kx = ky = kz = k.