In general, spectroscopy is the science of studying the interaction between matter and radiated energy, while spectrometry is the method used to acquire a quantitative spectrum measurement. Spectroscopy (scopy means observation) does not generate any results, and it is the theoretical approach of science. Spectrometry (metry means measurement) is the practical application where the results are generated. It measures the intensity of the radiation using an electronic device. Often these terms are used interchangeably, but every spectrometry is not spectroscopy (e.g., mass spectrometry vs. mass spectroscopy).
Gamma Spectroscopy
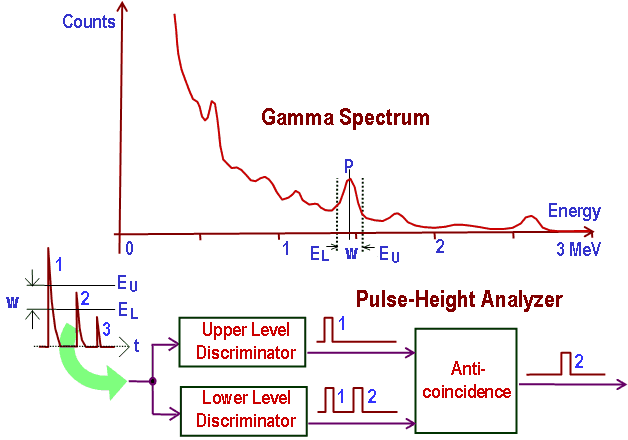
In general, gamma spectroscopy studies the energy spectra of gamma-ray sources, such as in the nuclear industry, geochemical investigation, and astrophysics. Spectroscopes, or spectrometers, are sophisticated devices designed to measure the spectral power distribution of a source. The incident radiation generates a signal that allows determining the energy of the incident particle.
Most radioactive sources produce gamma rays of various energies and intensities. Gamma rays frequently accompany the emission of alpha and beta radiation. A gamma-ray energy spectrum can be produced when these emissions are detected and analyzed with a spectroscopy system. Gamma rays from radioactive decay are in the energy range from a few keV to ~8 MeV, corresponding to the typical energy levels in nuclei with reasonably long lifetimes. As was written, they are produced by nuclei decay as they transition from a high energy state to a lower state. A detailed spectrum analysis is typically used to determine the identity and quantity of gamma emitters present in a sample. It is a vital tool in the radiometric assay. The gamma spectrum is characteristic of the gamma-emitting nuclides contained in the source.
X-Ray Spectroscopy
X-ray spectroscopy is a general term for several spectroscopic techniques for the characterization of materials by using x-ray excitation. When an electron from the inner shell of an atom is excited by the energy of a photon, it moves to a higher energy level. Since the process leaves a vacancy in the electron energy level from which the electron came, the outer electrons of the atom cascade down to fill the lower atomic levels, and one or more characteristic X-rays are usually emitted. As a result, sharp intensity peaks appear in the spectrum at wavelengths characteristic of the material from which the anode target is made. The frequencies of the characteristic X-rays can be predicted from the Bohr model. Analysis of the X-ray emission spectrum produces qualitative results about the elemental composition of the specimen.