 Ionization is the process in which an atom or a molecule gains or loses electrons to form a charged ion. Ionization can result from losing an electron after collisions with energetic subatomic particles, collisions with other atoms, molecules, and ions, or through the interaction with electromagnetic radiation. In general, ionizing radiation is any radiation (particles or electromagnetic waves) that carries enough energy to knock electrons from atoms or molecules, thereby ionizing them. For ionizing radiation, the kinetic energy of particles (photons, electrons, etc.) is sufficient, and the particle can ionize (to form ions by losing electrons) to target atoms to form ions.
Ionization is the process in which an atom or a molecule gains or loses electrons to form a charged ion. Ionization can result from losing an electron after collisions with energetic subatomic particles, collisions with other atoms, molecules, and ions, or through the interaction with electromagnetic radiation. In general, ionizing radiation is any radiation (particles or electromagnetic waves) that carries enough energy to knock electrons from atoms or molecules, thereby ionizing them. For ionizing radiation, the kinetic energy of particles (photons, electrons, etc.) is sufficient, and the particle can ionize (to form ions by losing electrons) to target atoms to form ions.
The boundary between ionizing and non-ionizing radiation is not sharply defined, since different molecules and atoms ionize at different energies. Gamma rays, X-rays, and the higher ultraviolet part of the spectrum are ionizing, whereas the lower ultraviolet, visible light (including laser light), infrared, microwaves, and radio waves are considered non-ionizing radiation.
Ionization Energy
Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.
X + energy → X+ + e−
where X is any atom or molecule capable of being ionized, X+ is that atom or molecule with an electron removed (positive ion), and e− is the removed electron.
A nitrogen atom, for example, requires the following ionization energy to remove the outermost electron.
N + IE → N+ + e− IE = 14.5 eV
The ionization energy associated with removing the first electron is most commonly used. The nth ionization energy refers to the amount of energy required to remove an electron from the species with a charge of (n-1).
1st ionization energy
X → X+ + e−
2nd ionization energy
X+ → X2+ + e−
3rd ionization energy
X2+ → X3+ + e−
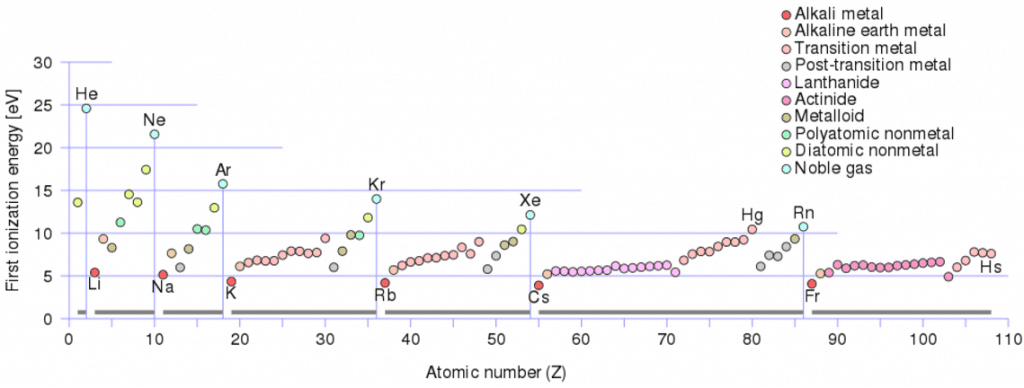
Ionization Energy for different Elements
There is ionization energy for each successive electron removed. The electrons that circle the nucleus move in fairly well-defined orbits. Some of these electrons are more tightly bound in the atom than others. For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88,000 eV is required to remove the innermost electron. Helps to understand reactivity of elements (especially metals, which lose electrons).
In general, the ionization energy increases moving up a group and left to the right across a period. Moreover:
- Ionization energy is lowest for the alkali metals, which have a single electron outside a closed shell.
- Ionization energy increases across a row on the periodic maximum for the noble gases, which have closed shells.
For example, sodium requires only 496 kJ/mol or 5.14 eV/atom to ionize it. On the other hand neon, the noble gas, immediately preceding it in the periodic table, requires 2081 kJ/mol or 21.56 eV/atom.