To understand the difference between metals, semiconductors and electrical insulators, we have to define the following terms from solid-state physics:
 Valence Band
Valence Band
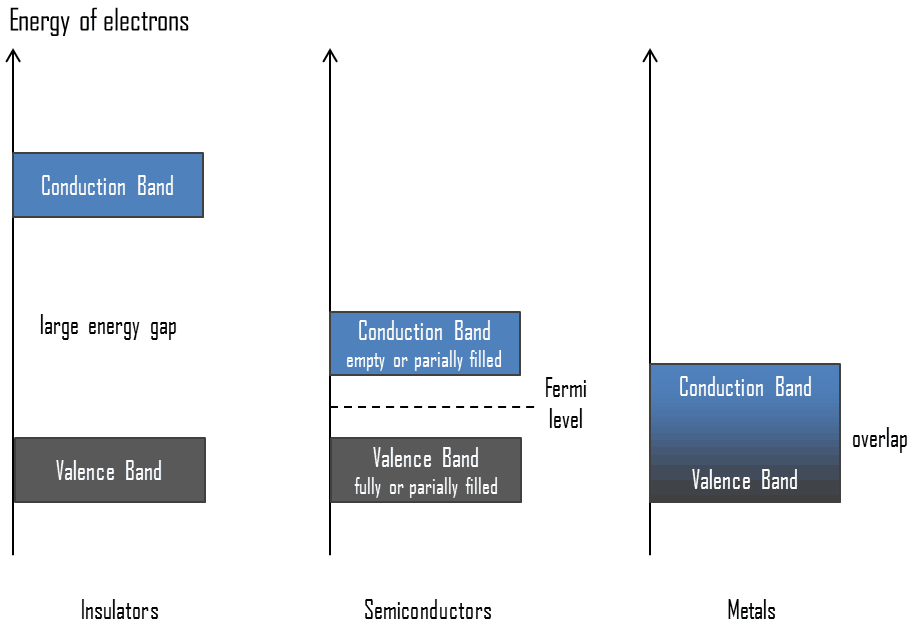
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In electrical insulators and semiconductors, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature. For example, a silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. The distinction between the valence and conduction bands is meaningless in metals because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.
Conduction Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. The conduction band is the lowest range of vacant electronic states in electrical insulators and semiconductors. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is above it. In semiconductors, electrons may reach the conduction band when they are excited, for example, by ionizing radiation (i.e., they must obtain energy higher than Egap). For example, diamond is a wide-bandgap semiconductor (Egap = 5.47 eV) with high potential as an electronic device material in many devices. On the other side, germanium has a small band gap energy (Egap = 0.67 eV), which requires operating the detector at cryogenic temperatures. The distinction between the valence and conduction bands is meaningless in metals because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.