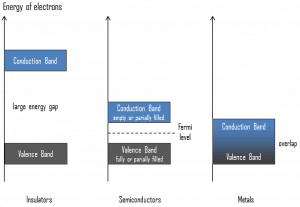
 In general, semiconductors are inorganic or organic materials that can control their conduction depending on chemical structure, temperature, illumination, and the presence of dopants. The name semiconductor comes from the fact that these materials have electrical conductivity between a metal, like copper, gold, etc., and an insulator, like glass. They have an energy gap of less than 4eV (about 1eV). In solid-state physics, this energy gap or band gap is an energy range between the valence and conduction bands where electron states are forbidden. In contrast to conductors, semiconductors’ electrons must obtain energy (e.g., from ionizing radiation) to cross the band gap and reach the conduction band.
In general, semiconductors are inorganic or organic materials that can control their conduction depending on chemical structure, temperature, illumination, and the presence of dopants. The name semiconductor comes from the fact that these materials have electrical conductivity between a metal, like copper, gold, etc., and an insulator, like glass. They have an energy gap of less than 4eV (about 1eV). In solid-state physics, this energy gap or band gap is an energy range between the valence and conduction bands where electron states are forbidden. In contrast to conductors, semiconductors’ electrons must obtain energy (e.g., from ionizing radiation) to cross the band gap and reach the conduction band.
Optical and Thermal Excitation in Semiconductors
Energy for the excitation can be obtained in different ways.
Thermal Excitation
Electron-hole pairs are also constantly generated from thermal energy without any external energy source. Thermal excitation does not require any other form of starting impulse. This phenomenon also occurs at room temperature. It is caused by impurities, irregularity in structure lattice, or by dopant. It strongly depends on the Egap (a distance between valence and conduction band), so that for lower Egap, the number of thermally excited charge carriers increases. Since thermal excitation results in the detector noise, active cooling is required for some types of semiconductors (e.g., germanium). Detectors based on silicon have sufficiently low noise even at room temperature. This is caused by the large band gap of silicon (Egap= 1.12 eV), which allows us to operate the detector at room temperature, but cooling is preferred to reduce noise.
Optical Excitation
Note that the energy of a single photon of the visible light spectrum is comparable with these band gaps. Photons of wavelengths 700 nm – 400 nm have energies of 1.77 eV to 3.10 eV. As a result, visible light can also excite electrons to the conduction band, which is the principle of photovoltaic panels that generate electric currents.