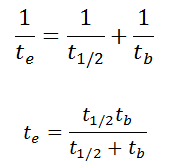A whole-body counter is an instrument that measures the amounts of gamma-emitting radionuclides in the body (i.e., it is a gamma spectrometer). In nuclear facilities, these counters are used for the measurement of radioactivity within the human body, that means, for internal contamination measurements. This must not be confused with a “whole body monitor,” which is used for personnel exit monitoring, which is the term used in radiation protection for checking for external contamination of the whole body of a person leaving radioactive contamination controlled area. Whole-body counters are very sensitive devices and are often surrounded by large quantities of lead shielding to reduce the background radiation. A whole-body counter consists, for example, of a stand-up booth with two large-area NaI scintillation detectors. The upper detector monitors the lungs, and the lower detector monitors the gastrointestinal tract.
It must be noted that all people also have some radioactive isotopes inside their bodies from birth. These isotopes are potassium-40, carbon-14, and the isotopes of uranium and thorium. The average annual radiation dose to a person from internal radioactive materials other than radon is about 0.3 mSv/year, which:
- 2 mSv/year comes from potassium-40,
- 12 mSv/year comes from the uranium and thorium series,
- 12 μSv/year comes from carbon-40.
The variation in radiation dose from one person to another is not as great, but it is also detected by a whole-body counter.
Gamma Spectroscopy

If a gamma ray is emitted from a radioactive element within the human body due to radioactive decay, and its energy is sufficient to escape, it can be detected. This would be using a gamma spectrometer. Spectroscopes, or spectrometers, are sophisticated devices designed to measure the spectral power distribution of a source. The incident radiation generates a signal that allows determining the energy of the incident particle. Most radioactive sources produce gamma rays of various energies and intensities. Gamma rays frequently accompany the emission of alpha and beta radiation. A gamma-ray energy spectrum can be produced when these emissions are detected and analyzed with a spectroscopy system. Gamma rays from radioactive decay are in the energy range from a few keV to ~8 MeV, corresponding to the typical energy levels in nuclei with reasonably long lifetimes. As was written, they are produced by nuclei decay as they transition from a high energy state to a lower state. A detailed spectrum analysis is typically used to determine the identity and quantity of gamma emitters present in a sample. It is a vital tool in the radiometric assay. The gamma spectrum is characteristic of the gamma-emitting nuclides contained in the source.
For the measurement of gamma rays above several hundred keV, there are two detector categories of major importance, inorganic scintillators such as NaI(Tl) and semiconductor detectors. In the previous articles, we described gamma spectroscopy using a scintillation detector, which consists of a suitable scintillator crystal, a photomultiplier tube, and a circuit for measuring the height of the pulses produced by the photomultiplier. The advantages of a scintillation counter are its efficiency (large size and high density) and the possible high precision and counting rates. Due to the high atomic number of iodine, a large number of all interactions will result in complete absorption of gamma-ray energy so that the photo fraction will be high.
But if a perfect energy resolution is required, we must use a germanium-based detector, such as the HPGe detector. Germanium-based semiconductor detectors are most commonly used where a very good energy resolution is required, especially for gamma spectroscopy as well as x-ray spectroscopy. In gamma spectroscopy, germanium is preferred due to its atomic number being much higher than silicon, increasing the probability of gamma-ray interaction. Moreover, germanium has lower average energy necessary to create an electron-hole pair, which is 3.6 eV for silicon and 2.9 eV for germanium. This also provides the latter with a better resolution in energy. The FWHM (full width at half maximum) for germanium detectors is a function of energy. For a 1.3 MeV photon, the FWHM is 2.1 keV, which is very low.
Internal Dose Uptake
If the radiation source is inside our body, we say it is internal exposure. The intake of radioactive material can occur through various pathways such as ingesting radioactive contamination in food or liquids, inhalation of radioactive gases, or through intact or wounded skin. Most radionuclides will give you much more radiation dose if they can somehow enter your body than they would if they remained outside. For internal doses, we first should distinguish between intake and uptake. Intake means what a person takes in, and uptake means what a person keeps.
When a radioactive compound enters the body, the activity will decrease with time due both to radioactive decay and biological clearance. The decrease varies from one radioactive compound to another. For this purpose, the biological half-life is defined in radiation protection.
The biological half-life is the time taken for the amount of a particular element in the body to decrease to half of its initial value due to elimination by biological processes alone when the removal rate is roughly exponential. The biological half-life depends on the rate at which the body normally uses a particular compound of an element. Radioactive isotopes that were ingested or taken in through other pathways will gradually be removed from the body via bowels, kidneys, respiration, and perspiration. This means that a radioactive substance can be expelled before it has had the chance to decay.
As a result, the biological half-life significantly influences the effective half-life and the overall dose from internal contamination. If a radioactive compound with a radioactive half-life (t1/2) is cleared from the body with a biological half-life tb, the effective half-life (te) is given by the expression:
As can be seen, the biological mechanisms always decrease the overall dose from internal contamination. Moreover, if t1/2 is large compared to tb, the effective half-life is approximately the same as tb.
For example, tritium has a biological half-life of about 10 days, while the radioactive half-life is about 12 years. On the other hand, radionuclides with very short radioactive half-lives have also very short effective half-lives. These radionuclides will deliver, for all practical purposes, the total radiation dose within the first few days or weeks after intake.
For tritium, the annual limit intake (ALI) is 1 x 109 Bq. Taking 1 x 109 Bq of tritium will receive a whole-body dose of 20 mSv. The committed effective dose, E(t), is 20 mSv. It does not depend on whether a person intakes this amount of activity in a short or long time. In every case, this person gets the same whole-body dose of 20 mSv.