A thermoluminescent dosimeter, abbreviated as TLD, is a passive radiation dosimeter that measures ionizing radiation exposure by measuring the intensity of visible light emitted from a sensitive crystal in the detector when the crystal is heated. The intensity of light emitted is measured by the TLD reader, depending on the radiation exposure. Thermoluminescent dosimeters were invented in 1954 by Professor Farrington Daniels of the University of Wisconsin-Madison. TLD dosimeters apply to situations where real-time information is not needed. Still, precise accumulated dose monitoring records are desired for comparison to field measurements or for assessing the potential for long-term health effects. In dosimetry, the quartz fiber and film badge types are superseded by TLDs and EPDs (Electronic Personal Dosimeter).
TLD – Principle of Operation
The following basic overview explains how a TLD works:
- When ionizing radiation passes through the detector (chip), the chip absorbs the radiation, and its structure changes slightly.
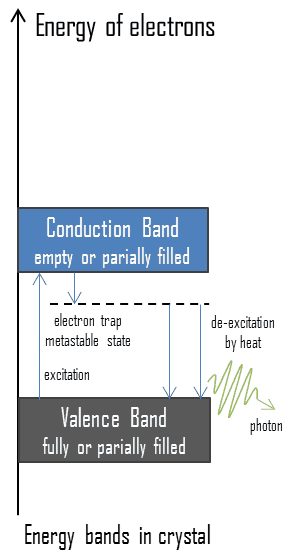
- In thermoluminescent materials, electrons may reach the conduction band when they are excited, for example, by ionizing radiation (i.e., they must obtain energy higher than Egap). But in this case, defects in the material’s existence or impurities are added to trap electrons in the band gap and hold them there.
- These trapped electrons represent stored energy for the time the electrons are held, and the amount of this energy depends upon the radiation exposure.
- The TLD chip must be heated in this TLD reader to obtain the dose received. The trapped electrons return to the ground state and emit photons of visible light. The amount of light emitted relative to the temperature is called the glow curve.
- After the readout is complete, the TLD is annealed at a high temperature. This process essentially zeroes the TL material by releasing all trapped electrons. The TLD is then ready for reuse.
TLD Reader
As was written, previously absorbed energy from electromagnetic radiation or other ionizing radiation in these materials is re-emitted as light upon heating the material. The intensity of light emitted is measured by the TLD reader, depending on the radiation exposure. A typical basic TLD reader contains the following components:
- Heater. Heater raises the temperature of the TL material
- Photomultiplier tube. PMT amplifies and measures the light output.
- Meter/Recorder. The recorder can display and record data.
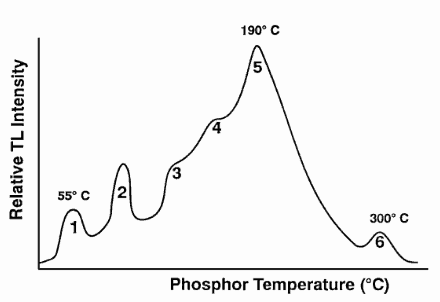
The TLD chip must be heated in this TLD reader to obtain the dose received. The trapped electrons return to the ground state and emit photons of visible light. The amount of light emitted relative to the temperature is called the glow curve. This curve is analyzed to determine the dose. After the readout is complete, the TLD is annealed at a high temperature. This process essentially zeroes the TL material by releasing all trapped electrons. The TLD is then ready for reuse. There are two types of readers. Automatic and manual readers. The automatic TLD reader is a lot more complicated than it might be expected.
Advantages and Disadvantages of TLDs
Advantages of TLDs
- TLDs can measure a greater range of doses in comparison with film badges.
- Doses from TLDs may be easily obtained.
- TLDs can be read on site instead of being sent away for development.
- TLDs are easily reusable.
Disadvantages of TLDs
- Each dose cannot be read out more than once.
- The readout process effectively “zeroes” the TLD.
Neutron Thermoluminescent Dosimeter – Neutron TLD
The personnel neutron dosimetry continues to be one of the problems in the field of radiation protection, as no single method provides the combination of energy response, sensitivity, orientation dependence characteristics, and accuracy necessary to meet the needs of a personnel dosimeter.
The most commonly used personnel neutron dosimeters for radiation protection purposes are thermoluminescent dosimeters and albedo dosimeters. Both are based on this phenomenon – thermoluminescence. For this purpose, lithium fluoride (LiF) as sensitive material (chip) is widely used. Lithium fluoride TLD is used for gamma and neutron exposure (indirectly, using the Li-6 (n, alpha)) nuclear reaction. Small crystals of LiF (lithium fluoride) are the most common TLD dosimeters since they have the same absorption properties as soft tissue. Lithium has two stable isotopes, lithium-6 (7.4 %) and lithium-7 (92.6 %). Li-6 is the isotope sensitive to neutrons. LiF crystal dosimeters may be enriched in lithium-6 to enhance the lithium-6 (n, alpha) nuclear reaction to record neutrons. The efficiency of the detector depends on the energy of the neutrons. Because the interaction of neutrons with any element is highly dependent on energy, making a dosimeter independent of the energy of neutrons is very difficult. LiF dosimeters are mostly utilized, containing different percentages of lithium-6 to separate thermal neutrons and photons. LiF chip enriched in lithium-6, which is very sensitive to thermal neutrons, and LiF chip containing very little lithium-6, which has a negligible neutron response.
The principle of neutron TLDs is then similar to gamma radiation TLDs. In the LiF chip, there are impurities (e.g., manganese or magnesium), which produce trap states for energetic electrons. The impurity causes traps in the crystalline lattice where electrons are held following irradiation (to alpha radiation). When the crystal is warmed, the trapped electrons are released, and light is emitted. The amount of light is related to the dose of radiation received by the crystal.
Thermoluminescent Albedo Neutron Dosimeter
Albedo neutron dosimetry is based on the human body’s effect of moderation and backscattering of neutrons. Albedo, the Latin word for “whiteness,” is defined by Lambert as the fraction of the incident light reflected diffusely by a surface. Moderation and backscattering of neutrons by the human body create a neutron flux at the body surface in the thermal and intermediate energy range. These backscattered neutrons, called albedo neutrons, can be detected by a dosimeter (usually a LiF TLD chip) placed on the body, designed to detect thermal neutrons. Albedo dosimeters are the only dosimeters that can measure doses due to neutrons over the whole range of energies. Usually, two types of lithium fluoride are used to separate doses contributed by gamma-rays and neutrons. LiF chip enriched in lithium-6, which is very sensitive to thermal neutrons, and LiF chip containing very little lithium-6, which has a negligible neutron response.
Radiation Dose Measuring and Monitoring
In previous chapters, we described the equivalent dose and the effective dose. But these doses are not directly measurable. For this purpose, the ICRP has introduced and defined a set of operational quantities that can be measured and intended to provide a reasonable estimate for the protection quantities. These quantities aim to provide a conservative estimate for the value of the protection quantities related to an exposure avoiding both underestimation and too much overestimation.
Numerical links between these quantities are represented by conversion coefficients, which are defined for a reference person. An internationally agreed set of conversion coefficients must be available for general use in radiological protection practice for occupational exposures and exposures of the public. Computational phantoms are used for dose assessment in various radiation fields to calculate conversion coefficients for external exposure. Biokinetic models for radionuclides, reference physiological data, and computational phantoms are used for calculating dose coefficients from intakes of radionuclides.
A set of evaluated data of conversion coefficients for protection and operational quantities for external exposure to a mono-energetic photon, neutron, and electron radiation under specific irradiation conditions is published in reports (ICRP, 1996b, ICRU, 1997).
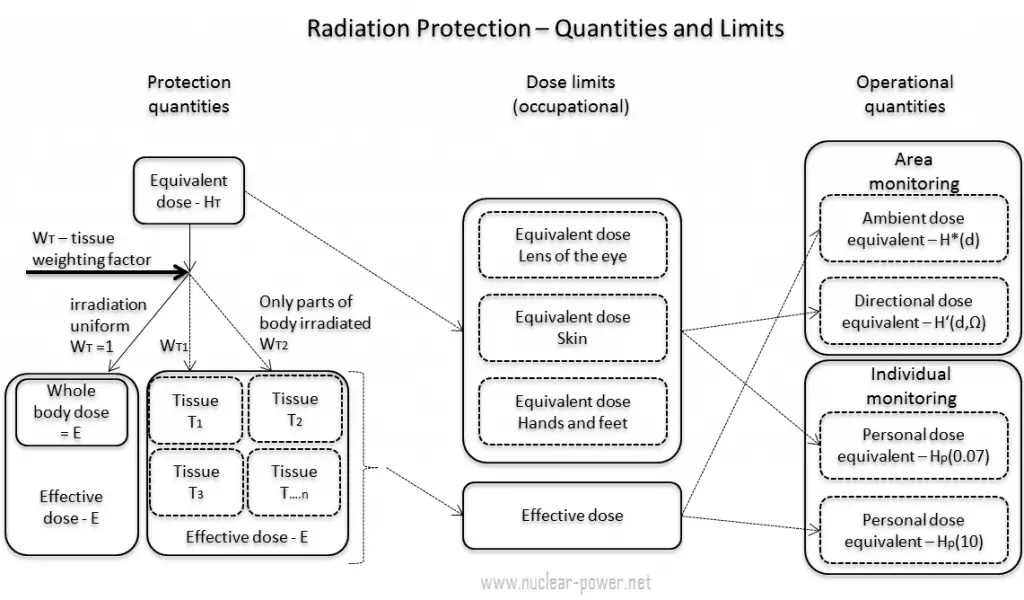
 In general, the ICRP defines operational quantities for the area and individual monitoring of external exposures. The operational quantities for area monitoring are:
In general, the ICRP defines operational quantities for the area and individual monitoring of external exposures. The operational quantities for area monitoring are:
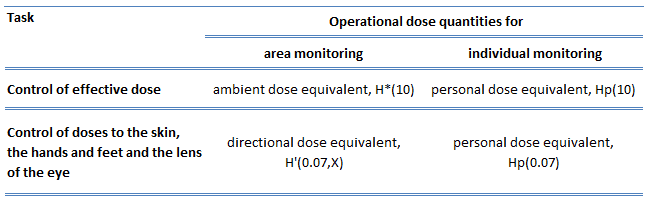
- Ambient dose equivalent, H*(10). The ambient dose equivalent is an operational quantity for area monitoring of strongly penetrating radiation.
- Directional dose equivalent, H’ (d, Ω). The directional dose equivalent is an operational quantity for area monitoring weakly penetrating radiation.
The operational quantities for individual monitoring are:
- Personal dose equivalent, Hp(0.07). The Hp(0.07) dose equivalent is an operational quantity for individual monitoring to assess the dose to the skin, hands, and feet.
- Personal dose equivalent, Hp(10). The Hp(10) dose equivalent is an operational quantity for individual monitoring to assess the effective dose.
Special Reference: ICRP, 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37 (2-4).
Dose Limits
See also: Dose Limits
Dose limits are split into two groups, the public and occupationally exposed workers. According to ICRP, occupational exposure refers to all exposure incurred by workers in the course of their work, except for
- excluded exposures and exposures from exempt activities involving radiation or exempt sources
- any medical exposure
- the normal local natural background radiation.
The following table summarizes dose limits for occupationally exposed workers and the public:
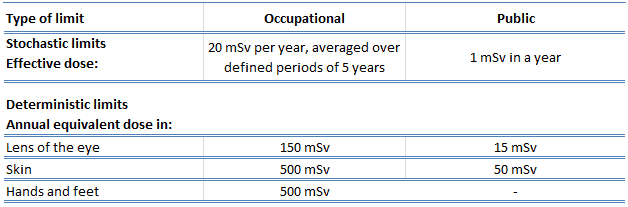
Source of data: ICRP, 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37 (2-4).
According to the recommendation of the ICRP in its statement on tissue reactions of 21. April 2011, the equivalent dose limit for the eye lens for occupational exposure in planned exposure situations was reduced from 150 mSv/year to 20 mSv/year, averaged over defined periods of 5 years, with no annual dose in a single year exceeding 50 mSv.
Limits on effective dose are for the sum of the relevant, effective doses from external exposure in the specified period and the committed effective dose from intakes of radionuclides in the same period. For adults, the committed effective dose is computed for 50 years after intake, whereas for children, it is computed for the period up to 70 years. The effective whole-body dose limit of 20 mSv is an average value over five years, and the real limit is 100 mSv in 5 years, with no more than 50 mSv in any year. Toward that end, employers carefully monitor the exposure of these individuals using instruments called dosimeters worn on a position of the body representative of its exposure. In most situations of occupational exposure, the effective dose, E, can be derived from operational quantities using the following formula:

Sievert – Unit of Equivalent Dose
In radiation protection, a sievert is a derived unit of equivalent dose and effective dose. The sievert represents the equivalent biological effect of depositing a joule of gamma rays energy in a kilogram of human tissue. Unit of sievert is important in radiation protection and was named after the Swedish scientist Rolf Sievert, who did a lot of the early work on radiation dosimetry in radiation therapy.
As was written, the sievert is used for radiation dose quantities such as equivalent dose and effective dose. Equivalent dose (symbol HT) is a dose quantity calculated for individual organs (index T – tissue). The equivalent dose is based on the absorbed dose to an organ, adjusted to account for the effectiveness of the type of radiation. An equivalent dose is given the symbol HT. The SI unit of HT is the sievert (Sv) or but rem (roentgen equivalent man) is still commonly used (1 Sv = 100 rem).
Examples of Doses in Sieverts
We must note that radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us, and it is a part of our natural world that has been here since the birth of our planet. In the following points, we try to express enormous ranges of radiation exposure, which can be obtained from various sources.
- 0.05 µSv – Sleeping next to someone
- 0.09 µSv – Living within 30 miles of a nuclear power plant for a year
- 0.1 µSv – Eating one banana
- 0.3 µSv – Living within 50 miles of a coal power plant for a year
- 10 µSv – Average daily dose received from natural background
- 20 µSv – Chest X-ray
- 40 µSv – A 5-hour airplane flight
- 600 µSv – mammogram
- 1 000 µSv – Dose limit for individual members of the public, total effective dose per annum
- 3 650 µSv – Average yearly dose received from natural background
- 5 800 µSv – Chest CT scan
- 10 000 µSv – Average yearly dose received from a natural background in Ramsar, Iran
- 20 000 µSv – single full-body CT scan
- 175 000 µSv – Annual dose from natural radiation on a monazite beach near Guarapari, Brazil.
- 5 000 000 µSv – Dose that kills a human with a 50% risk within 30 days (LD50/30) if the dose is received over a very short duration.