Radiation biology (also known as radiobiology) is a medical science that studies the biological effects of ionizing radiation on living tissues. Radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us, and it is a part of our natural world that has been here since the birth of our planet. Whether the source of radiation is natural or manufactured, whether it is a large dose of radiation or a small dose, there will be some biological effects. In general, ionizing radiation is harmful and potentially lethal to living beings. Still, it can have health benefits in medicine, for example, in radiation therapy to treat cancer and thyrotoxicosis. This chapter briefly summarizes the short- and long-term consequences of exposure to radiation.
Cellular Damage – Radiobiology
All biological damage effects begin with the consequence of radiation interactions with the atoms forming the cells. All living things are composed of one or more cells, and every part of your body consists of cells or was built by them. Although we tend to think of biological effects in terms of the effect of radiation on living cells, in actuality, ionizing radiation, by definition, interacts only with atoms by a process called ionization. The kinetic energy of particles (photons, electrons, etc.) of ionizing radiation is sufficient for ionizing radiation. The particle can ionize (to form ions by losing electrons) target atoms to form ions, and ionizing radiation can knock electrons from an atom.
There are two mechanisms by which radiation ultimately affects cells. These two mechanisms are commonly called:
- Direct effects. Direct effects are caused by radiation when radiation interacts directly with the atoms of the DNA molecule or some other cellular component critical to the survival of the cell. The probability of the radiation interacting with the DNA molecule is very small since these critical components make up such a small part of the cell.
- Indirect effects. Indirect effects are caused by the interaction of radiation, usually with water molecules. Each cell, just as is the case for the human body, is mostly water. Ionizing radiation may break the bonds that hold the water molecule together, producing radicals such as hydroxyl OH, superoxide anion O2–and others. These radicals can contribute to the destruction of the cell.
A large number of cells of any particular type is called a tissue. If this tissue forms a specialized functional unit, it is called an organ. The type and number of cells affected is also important factor. Some cells and organs in the body are more sensitive to ionizing radiation than others.
The sensitivity of various types of cells to ionizing radiation is very high for tissues consisting of cells that divide rapidly like those found in bone marrow, stomach, intestines, male and female reproductive organs, and developing fetuses. This is because dividing cells require correct DNA information for the cell’s offspring to survive. Direct interaction of radiation with an active cell could result in the death or mutation of the cell, whereas a direct interaction with the DNA of a dormant cell would have less of an effect.
As a result, living cells can be classified according to their reproduction rate, indicating their relative sensitivity to radiation. As a result, actively reproducing cells are more sensitive to ionizing radiation than cells that make up skin, kidney, or liver tissue. The nerve and muscle cells are the slowest to regenerate and are the least sensitive cells.
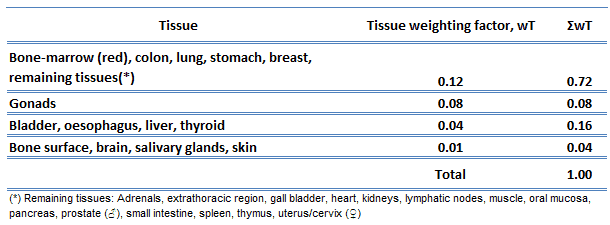 The sensitivity of the various organs of the human body correlates with the relative sensitivity of the cells from which they are composed. In practice, this sensitivity is represented by the tissue weighting factor, wT, which is the factor by which the equivalent dose in a tissue or organ T is weighted to represent the relative contribution of that tissue or organ to the total health detriment resulting from uniform irradiation of the body (ICRP 1991b).
The sensitivity of the various organs of the human body correlates with the relative sensitivity of the cells from which they are composed. In practice, this sensitivity is represented by the tissue weighting factor, wT, which is the factor by which the equivalent dose in a tissue or organ T is weighted to represent the relative contribution of that tissue or organ to the total health detriment resulting from uniform irradiation of the body (ICRP 1991b).
If a person is irradiated only partially, the dose will depend strongly on the irradiated tissue. For example, a 10 mSv gamma dose to the whole body and a 50 mSv dose to the thyroid is the same, in terms of risk, as a whole-body dose of 10 + 0.04 x 50 = 12 mSv.
High-LET and Low-LET Radiation
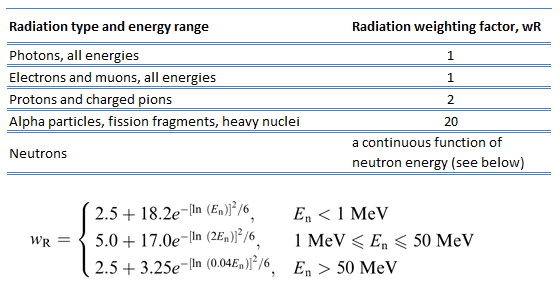
As was written, each type of radiation interacts with matter in a different way. For example, charged particles with high energies can directly ionize atoms. Alpha particles are fairly massive and carry a double positive charge, so they tend to travel only a short distance and do not penetrate very far into a tissue, if at all. However, alpha particles will deposit their energy over a smaller volume (possibly only a few cells if they enter a body) and cause more damage to those few cells.
Beta particles (electrons) are much smaller than alpha particles and carry a single negative charge. They are more penetrating than alpha particles and can travel several meters but deposit less energy at any point along their paths than alpha particles. This means beta particles tend to damage more cells but with lesser damage. On the other hand, electrically neutral particles interact indirectly but can also transfer some or all of their energies to the matter.
It would certainly simplify matters if the biological effects of radiation were directly proportional to the absorbed dose. Unfortunately, biological effects also depend on how the absorbed dose is distributed along the radiation path. Studies have shown that alpha and neutron radiation cause greater biological damage for a given energy deposition per kg of tissue than gamma radiation does. Biological effects of any radiation increase with the linear energy transfer (LET) were discovered. In short, the biological damage from high-LET radiation (alpha particles, protons, or neutrons) is much greater than that from low-LET radiation (gamma rays). This is because the living tissue can more easily repair damage from radiation spread over a large area than concentrated in a small area. Of course, at very high levels of exposure, gamma rays can still cause a great deal of damage to tissues.
Because more biological damage is caused for the same physical dose (i.e., the same energy deposited per unit mass of tissue), one gray of alpha or neutron radiation is more harmful than one gray of gamma radiation. The fact that radiations of different types (and energies) give different biological effects for the same absorbed dose is described in terms of factors known as the relative biological effectiveness (RBE) and the radiation weighting factor (wR).
Acute Dose and Chronic Dose
The biological effects of radiation and their consequences depend strongly on the level of dose rate obtained. In radiobiology, the dose rate measures radiation dose intensity (or strength). Low-level doses are common in everyday life. In the following points, a few examples of radiation exposure can be obtained from various sources.
- 05 µSv – Sleeping next to someone
- 09 µSv – Living within 30 miles of a nuclear power plant for a year
- 1 µSv – Eating one banana
- 3 µSv – Living within 50 miles of a coal power plant for a year
- 10 µSv – Average daily dose received from natural background
- 20 µSv – Chest X-ray
From biological consequences point of view, it is very important to distinguish between doses received over short and extended periods. Therefore, the biological effects of radiation are typically divided into two categories.
- Acute Doses. An “acute dose” (short-term high-level dose) occurs over a short and finite period, i.e., within a day.
- Chronic Doses. A “chronic dose” (long-term low-level dose) is a dose that continues for an extended period, i.e., weeks and months, so that a dose rate better describes it.
High doses tend to kill cells, while low doses tend to damage or change them. High doses can cause visually dramatic radiation burns and/or rapid fatality through acute radiation syndrome. Acute doses below 250 mGy are unlikely to have any observable effects. Acute doses of about 3 to 5 Gy have a 50% chance of killing a person some weeks after the exposure if a person receives no medical treatment.
Low doses spread out over long periods don’t cause an immediate problem to any body organ. The effects of low doses of radiation occur at the level of the cell, and the results may not be observed for many years. Moreover, some studies demonstrate that most human tissues exhibit a more pronounced tolerance to the effects of low-LET radiation in case of a prolonged exposure compared to a one-time exposure to a similar dose.
Deterministic and Stochastic Effects
In radiobiology, most adverse health effects of radiation exposure are usually divided into two broad classes:
- Deterministic effects are threshold health effects related directly to the absorbed radiation dose, and the severity of the effect increases as the dose increases.
- Stochastic effects occur by chance, generally occurring without a threshold level of dose. The probability of occurrence of stochastic effects is proportional to the dose, but the severity of the effect is independent of the dose received.
Deterministic Effects
In radiobiology, deterministic effects (or non-stochastic health effects) are health effects that are related directly to the absorbed radiation dose, and the severity of the effect increases as the dose increases. Deterministic effects have a threshold below which no detectable clinical effects do occur. The threshold may be very low (of the order of magnitude of 0.1 Gy or higher) and may vary from person to person. For doses between 0.25 Gy and 0.5 Gy, slight blood changes may be detected by medical evaluations, and for doses between 0.5 Gy and 1.5 Gy, blood changes will be noted. Symptoms of nausea, fatigue, and vomiting occur.
Once the threshold has been exceeded, the severity of an effect increases with the dose. The reason for the presence of this threshold dose is that radiation damage (serious malfunction or death) of a critical population of cells (high doses tend to kill cells) in a given tissue needs to be sustained before an injury is expressed in a clinically relevant form. Therefore, deterministic effects are also termed tissue reactions. They are also called non-stochastic effects to contrast with chance-like stochastic effects (e.g., cancer induction).
Deterministic effects are not necessarily more or less serious than stochastic effects. High doses can cause visually dramatic radiation burns and/or rapid fatality through acute radiation syndrome. Acute doses below 250 mGy are unlikely to have any observable effects. Acute doses of about 3 to 5 Gy have a 50% chance of killing a person some weeks after the exposure if a person receives no medical treatment. Deterministic effects can ultimately lead to a temporary nuisance or fatality. Examples of deterministic effects:
Examples of deterministic effects are:
- Acute radiation syndrome, by acute whole-body radiation
- Radiation burns, from radiation to a particular body surface
- Radiation-induced thyroiditis, a potential side effect of radiation treatment against hyperthyroidism
- Chronic radiation syndrome from long-term radiation.
- Radiation-induced lung injury, from, for example, radiation therapy to the lungs
Lethal Doses of Radiation
The lethal dose of radiation (LD) indicates the lethal amount of radiation. The median lethal dose, LDXY, is usually used in radiation protection. For example, the dose of radiation expected to cause death to 50 % of the irradiated persons within 30 days is LD50/30. LD1 is the dose expected to cause death to 1% of the irradiated persons. Consequently, LD99 is lethal for all (99%) persons irradiated. It is also very important whether a person receives some medical treatment or not. The greater an acute radiation dose is, the greater the possibility of it killing the individual. The LD50 is estimated to be between 3 and 5 Gy for a healthy adult.
- 2.5 Sv – Dose that kills a human with a 1% risk (LD1) if the dose is received over a very short duration.
- 5 Sv – Dose that kills a human with a 50% risk within 30 days (LD50/30) if the dose is received over a very short duration. The cause of death will be loss of bone marrow function.
- 8 Sv – Dose that kills a human with a 99% risk (LD99) if the dose is received over a very short duration. At around 10 Gy, acute inflammation of the lungs can occur and lead to death.
The lethal dose data above apply to acute gamma doses delivered in a very short time, e.g., a few minutes. More dose is required to produce the effects listed above if the dose is received for hours or longer.
Stochastic Effects
In radiobiology, stochastic effects of ionizing radiation occur by chance, generally occurring without a threshold level of dose. The probability of occurrence of stochastic effects is proportional to the dose, but the severity of the effect is independent of the dose received. The biological effects of radiation on people can be grouped into somatic and hereditary effects. Somatic effects are those suffered by the exposed person, and hereditary effects are those suffered by the offspring of the individual exposed. Cancer risk is usually mentioned as the main stochastic effect of ionizing radiation, but also hereditary disorders are stochastic effects.
According to ICRP:
(83) based on these calculations, the Commission proposes nominal probability coefficients for detriment-adjusted cancer risk as 5.5 x 10-2 Sv-1 for the whole population and 4.1 x 10-2 Sv-1 for adult workers. For heritable effects, the detriment-adjusted nominal risk in the whole population is estimated as 0.2 x 10-2 Sv-1 and in adult workers as 0.1 x 10-2 Sv-1.
Special Reference: ICRP, 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37 (2-4).
The SI unit for effective dose, the sievert, represents the equivalent biological effect of depositing a joule of gamma rays energy in a kilogram of human tissue. As a result, one sievert represents a 5.5% chance of developing cancer. Note that the effective dose is not intended as a measure of deterministic health effects, which is the severity of acute tissue damage that is certain to happen, measured by the quantity absorbed dose.
There are three general categories of stochastic effects resulting from exposure to low doses of radiation. These are:
- Genetic effects. The genetic effect is suffered by the offspring of the individual exposed. It involves the mutation of specific cells, namely the sperm or egg cells. Radiation is an example of a physical mutagenic agent. Note that there are also many chemical agents as well as biological agents (such as viruses) that cause mutations. One very important fact to remember is that radiation increases the spontaneous mutation rate but does not produce any new mutations.
- Somatic effects. Somatic effects are those suffered by the exposed person. The most common impact of irradiation is the stochastic induction of cancer with a latent period of years or decades after the exposure. Since cancer is the primary result, it is sometimes called the carcinogenic effect. Radiation is an example of a physical carcinogenic, while cigarettes are an example of a chemical cancer-causing agent, and viruses are examples of carcinogenic biological agents.
- In-Utero effects involve the production of malformations in developing embryos. However, this is a special case of the somatic effect since the embryo/fetus is the one exposed to the radiation.
Most thought somatic effects resulting from radiation exposure are thought to occur in a stochastic manner. The most widely accepted model posits that the incidence of cancers due to ionizing radiation increases linearly with effective radiation dose at a rate of 5.5% per sievert. This model is known as the linear no-threshold model (LNT). This model assumes no threshold point, and the risk increases linearly with a dose. If this linear model is correct, natural background radiation is the most hazardous radiation source to general public health, followed by medical imaging as a close second. The LNT is not universally accepted, with some proposing an adaptive dose-response relationship where low doses are protective, and high doses are detrimental. It must be emphasized that many organizations disagree with using the linear no-threshold model to estimate risk from environmental and occupational low-level radiation exposure.
Radiobiology and Dose Limits
In radiation protection, dose limits are set to limit stochastic effects to an acceptable level and prevent deterministic effects completely. Note that stochastic effects arise from chance: the greater the dose, the more likely the effect. Deterministic effects normally have a threshold: above this, the severity of the effect increases with the dose. Dose limits are a fundamental component of radiation protection, and breaching these limits is against radiation regulation in most countries. Note that the dose limits described in this article apply to routine operations. They do not apply to an emergency situation when human life is endangered. They do not apply in emergency exposure situations where an individual attempts to prevent a catastrophic situation.
The limits are split into two groups, the public and occupationally exposed workers. According to ICRP, occupational exposure refers to all exposure incurred by workers in the course of their work, except for
- excluded exposures and exposures from exempt activities involving radiation or exempt sources
- any medical exposure
- the normal local natural background radiation.
The following table summarizes dose limits for occupationally exposed workers and the public:
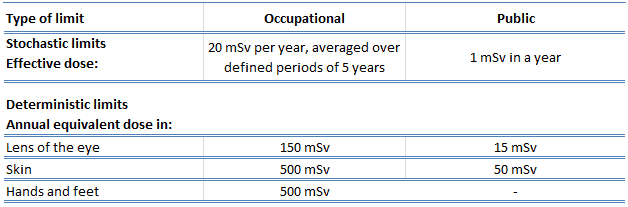
Source of data: ICRP, 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37 (2-4).
According to the recommendation of the ICRP in its statement on tissue reactions of 21. April 2011, the equivalent dose limit for the eye lens for occupational exposure in planned exposure situations was reduced from 150 mSv/year to 20 mSv/year, averaged over defined periods of 5 years, with no annual dose in a single year exceeding 50 mSv.
Limits on effective dose are for the sum of the relevant, effective doses from external exposure in the specified period and the committed effective dose from intakes of radionuclides in the same period. For adults, the committed effective dose is computed for a 50-year period after intake, whereas for children, it is computed for the period up to age 70. The effective whole-body dose limit of 20 mSv is an average value over five years, and the real limit is 100 mSv in 5 years, with not more than 50 mSv in any year.