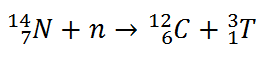Tritium is the only naturally-occurring radioisotope of hydrogen. Its atomic number is naturally 1, which means there is 1 proton and 1 electron in the atomic structure. Unlike the hydrogen nucleus and deuterium nucleus, tritium has 2 neutrons in the nucleus. Tritium is naturally-occurring, but it is extremely rare.
Tritium is produced in the atmosphere when cosmic rays collide with air molecules. In the most important reaction for natural production, a fast neutron (which must have energy greater than 4.0 MeV) interacts with atmospheric nitrogen:
Worldwide, the production of tritium from natural sources is 148 petabecquerels per year. As a result, the tritiated water produced participates in the water cycle.
- about 400 Bq/m3 in continental water
- about 100 Bq/m3 in oceans
Tritium is a radioactive isotope, but it emits a very weak form of radiation, a low-energy beta particle similar to an electron. It is a pure beta emitter (i.e., beta emitter without accompanying gamma radiation). The electron’s kinetic energy varies, with an average of 5.7 keV, while the remaining energy is carried off by the nearly undetectable electron antineutrino. Such a very low energy of electron causes that the electron cannot penetrate the skin or even does not travel very far in the air. Beta particles from tritium can penetrate only about 6.0 mm of air. Tritium decays via negative beta decay into helium-3 with a half-life of 12.3 years.
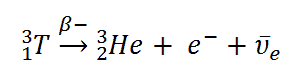 Therefore, tritium poses a risk to health due to internal exposure only the following ingestion in drinking water or food or inhalation or absorption through the skin. The tritium taken into the body is uniformly distributed among all soft tissues. An average annual dose from natural tritium intake is 0.01 μSv.
Therefore, tritium poses a risk to health due to internal exposure only the following ingestion in drinking water or food or inhalation or absorption through the skin. The tritium taken into the body is uniformly distributed among all soft tissues. An average annual dose from natural tritium intake is 0.01 μSv.
In the case of artificial tritium ingestion or inhalation, a biological half-time of tritium is 10 days for HTO and 40 days for OBT (organically bound tritium) formed from HTO in the body of adults. It was also shown that the biological half-time of HTO depends strongly on many variables and varies from about 4 to 18 days. During the warmer months, the average half-life is lower due to increased water intake. As well as, drinking larger amounts of alcohol will reduce the biological half-life of water in the body.