Radon is a colorless, odorless, tasteless noble gas, occurring naturally as the decay product of radium. Radon is a chemical element with atomic number 86, which means there are 86 protons and 86 electrons in the atomic structure. The chemical symbol for radon is Rn. All isotopes of radon are radioactive, but the two radon isotopes, radon-222 and radon-220, are very important from a radiation protection point of view.
-
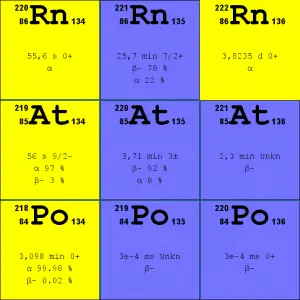
Source: JANIS (Java-based Nuclear Data Information Software); ENDF/B-VII.1 Radon-222. The radon-222 isotope is a natural decay product of the most stable uranium isotope (uranium-238). Thus it is a member of the uranium series.
- Radon-220. The radon-220 isotope, commonly referred to as thoron, is a natural decay product of the most stable thorium isotope (thorium-232). Thus it is a member of the thorium series.
It is important to note that radon is a noble gas, whereas all its decay products are metals. The main mechanism for radon entry into the atmosphere is diffusion through the soil. As a gas, radon diffuses through rocks and the soil. When radon disintegrates, the daughter metallic isotopes are ions that will be attached to other molecules like water and aerosol particles in the air. Therefore all discussions of radon concentrations in the environment refer to radon-222. While the average rate of production of radon-220 (thoron) is about the same as that of radon-222, the amount of radon-220 in the environment is much less than that of radon-222 because of the significantly shorter half-life (it has less time to diffuse) of radon-222 (55 seconds, versus 3.8 days respectively). Simply radon-220 has a lower chance of escaping from bedrock.
See also: Radon – Health Effects
Radon – Properties
| Element | Radon |
|---|---|
| Atomic Number | 86 |
| Symbol | Rn |
| Element Category | Noble Gas |
| Phase at STP | Gas |
| Atomic Mass [amu] | 222 |
| Density at STP [g/cm3] | 9.73 |
| Electron Configuration | [Hg] 6p6 |
| Possible Oxidation States | 0 |
| Electron Affinity [kJ/mol] | — |
| Electronegativity [Pauling scale] | — |
| 1st Ionization Energy [eV] | 10.7485 |
| Year of Discovery | 1900 |
| Discoverer | Dorn, Friedrich Ernst |
| Thermal properties | |
| Melting Point [Celsius scale] | -71 |
| Boiling Point [Celsius scale] | -61.8 |
| Thermal Conductivity [W/m K] | 0.00361 |
| Specific Heat [J/g K] | 0.09 |
| Heat of Fusion [kJ/mol] | 2.89 |
| Heat of Vaporization [kJ/mol] | 16.4 |
Atomic Mass of Radon
Atomic mass of Radon is 222 u.
Note that, each element may contain more isotopes. Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance.
The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10-24 grams. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
Density of Radon
Density of Radon is 9.73g/cm3.

Typical densities of various substances at atmospheric pressure.
Density is defined as the mass per unit volume. It is an intensive property, which is mathematically defined as mass divided by volume:
ρ = m/V
In words, the density (ρ) of a substance is the total mass (m) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/m3). The Standard English unit is pounds mass per cubic foot (lbm/ft3).
See also: What is Density
See also: Densest Materials of the Earth
Radon – Melting Point and Boiling Point
Melting point of radon is -71°C.
Boiling point of radon is -61.8°C.
Note that these points are associated with the standard atmospheric pressure.
Radon – Specific Heat, Latent Heat of Fusion, Latent Heat of Vaporization
Specific heat of radon is 0.09 J/g K.
Latent Heat of Fusion of Radon is 2.89 kJ/mol.
Latent Heat of Vaporization of Radon is 16.4 kJ/mol.