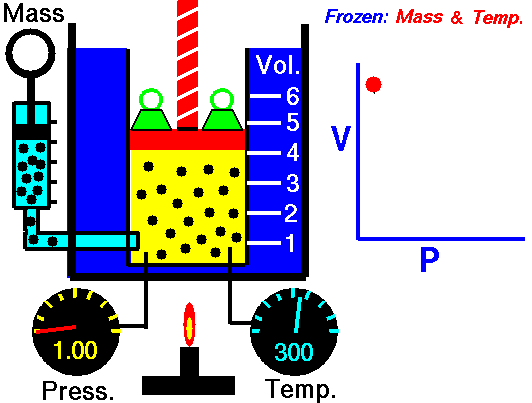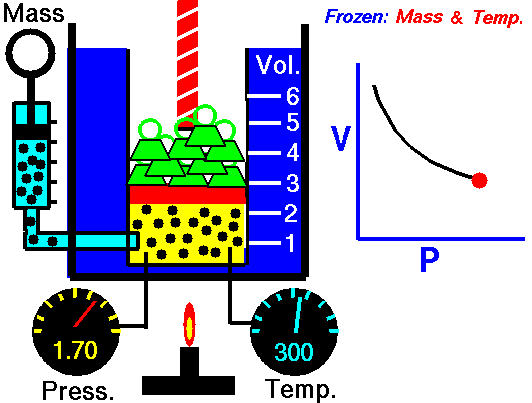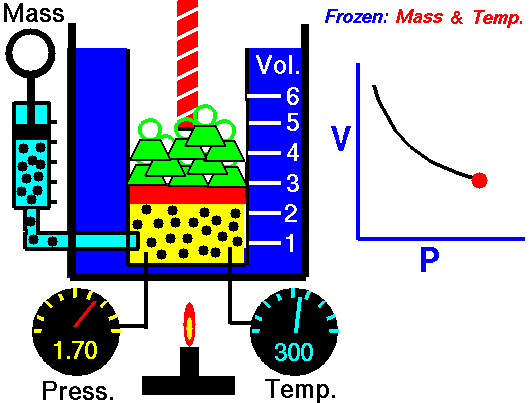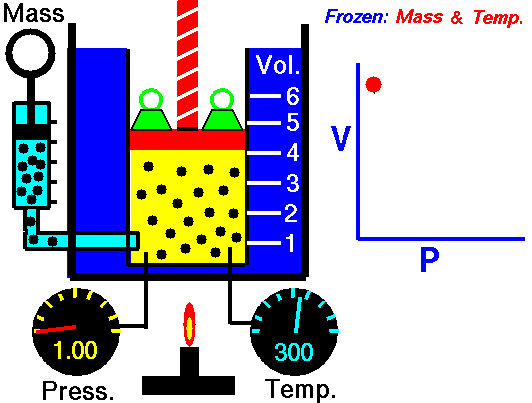
For a fixed mass of gas at a constant temperature, the volume is inversely proportional to the pressure.
Boyle-Mariotte Law is one of the gas laws. At the end of the 17th century, Robert William Boyle and Edme Mariotte independently studied the relationship between volume and pressure at a constant temperature. The results of certain experiments with gases at relatively low pressure led Robert Boyle to formulate a well-known law. It states that:
For a fixed mass of gas at a constant temperature, the volume is inversely proportional to the pressure.
That means that, for example, if you increase the volume 10 times, the pressure will decrease 10 times. If you halve the volume, you will double the pressure.
You can express this mathematically as:
pV = constant
or
p1V1 = p2V2
Yes, it seems to be identical to the isothermal process of an ideal gas. In fact, during their experiments, the temperature remained constant, as was assumed by Mariotte. These results are fully consistent with the ideal gas law, which determinates that the constant is equal to nRT.
pV = nRT
where:
- p is the absolute pressure of the gas
- n is the amount of substance
- T is the absolute temperature
- V is the volume
- R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
In this equation, the symbol R is the universal gas constant that has the same value for all gases—namely, R = 8.31 J/mol K.