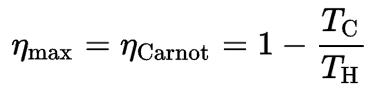Various Statements of the Law
The second law of thermodynamics may be expressed in many specific ways. Each statement expresses the same law. Listed below are three that are often encountered.
Before these statements, we have to remind the French engineer and physicist, Nicolas Léonard Sadi Carnot, advanced the study of the second law by forming a principle (also called Carnot’s rule) that specifies limits on the maximum efficiency any heat engine can obtain.
Clausius Statement of the Second Law
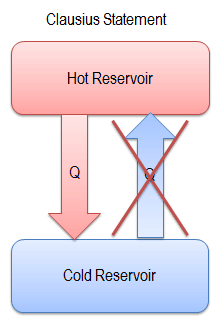 One of the earliest statements of the Second Law of Thermodynamics was made by R. Clausius in 1850. He stated the following.
One of the earliest statements of the Second Law of Thermodynamics was made by R. Clausius in 1850. He stated the following.
“It is impossible to construct a device which operates on a cycle and whose sole effect is the transfer of heat from a cooler body to a hotter body”.
Heat cannot spontaneously flow from cold system to hot system without external work being performed on the system. This is exactly what refrigerators and heat pumps accomplish. In a refrigerator, heat flows from cold to hot, but only when forced by external work. Refrigerators are driven by electric motors requiring work from their surroundings to operate.
The Clausius and the Kelvin-Planck statements have been shown to be equivalent.