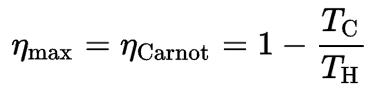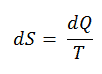Various Statements of the Law
The second law of thermodynamics may be expressed in many specific ways. Each statement expresses the same law. Listed below are three that are often encountered.
Before these statements, we have to remind the French engineer and physicist, Nicolas Léonard Sadi Carnot. They advanced the study of the second law by forming a principle (also called Carnot’s rule) that specifies limits on the maximum efficiency that any heat engine can obtain.
Entropy and the Second Law
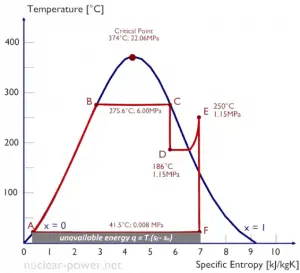
One consequence of the second law of thermodynamics is the development of the physical property of matter, which is known as the entropy (S). The change in this property is used to determine the direction in which a given process will proceed. Entropy quantifies the energy of a substance that is no longer available to perform useful work. This relates to the second law since the second law predicts that not all heat provided to a cycle can be transformed into an equal amount of work. Some heat rejection must take place.
See also: Entropy
According to Clausius, the entropy was defined via the change in entropy S of a system. The change in entropy S, when an amount of heat Q is added to it by a reversible process at a constant temperature, is given by:
Here Q is the energy transferred as heat to or from the system during the process, and T is the temperature of the system in kelvins during the process. The SI unit for entropy is J/K.
The second law of thermodynamics can also be expressed as ∆S≥0 for a closed cycle.
In words:
The entropy of an isolated system never decreases. In a natural thermodynamic process, the sum of the entropies of the interacting thermodynamic systems increases.
∆S≥0
Because entropy tells so much about the usefulness of an amount of heat transferred in performing work, the steam tables include values of specific entropy (s = S/m) as part of the information tabulated.